:
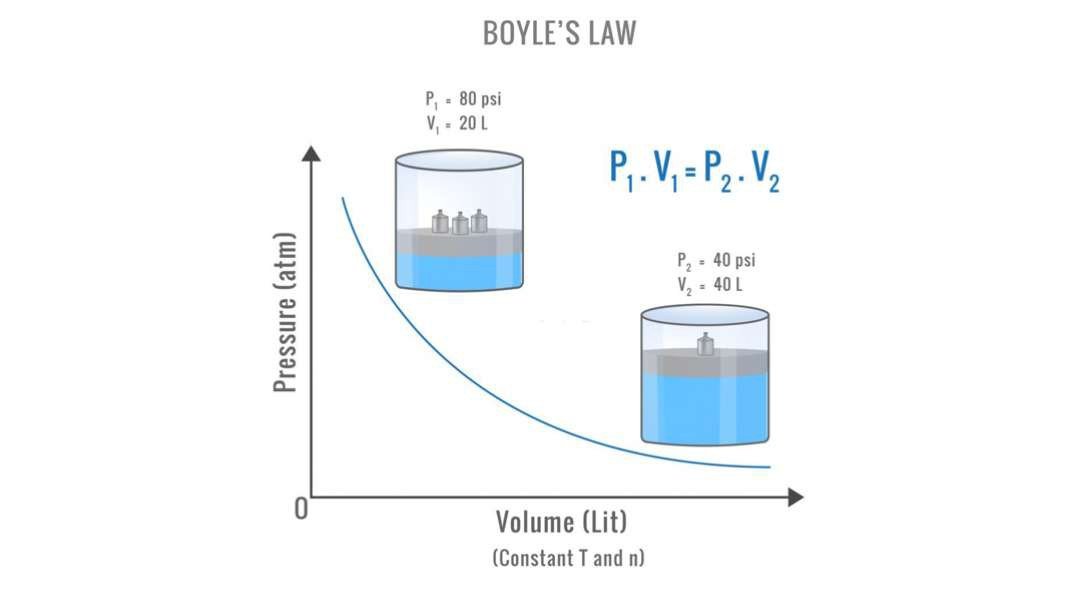
Boyle's Law
According to Boyle's Law, the pressure (P) of a given mass of gas is inversely proportional to its volume (V), provided that the temperature of the gas remains constant. For an enclosed gas, at constant temperature (T); or, ... The quill tube is helpful in verifying Boyle's law.
What is Boyle's gas law?
This empirical relation, formulated by the physicist Robert Boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form, pv = k, a constant.
What is Boyle's law in simple terms?
: a statement in physics: the volume of a gas at constant temperature varies inversely with the pressure exerted on it.
How many gas laws are there in class 11?
five gas laws
The five gas laws are: Boyle's Law, which provides a relationship between the pressure and the volume of a gas. Charles's Law, which provides a relationship between the volume occupied by a gas and the absolute temperature.
