:
Elements and Compounds
0
0
2 Views
0 Purchases
Published on 10 Jun 2022 / In
Chemistry / Chemistry Grade 9
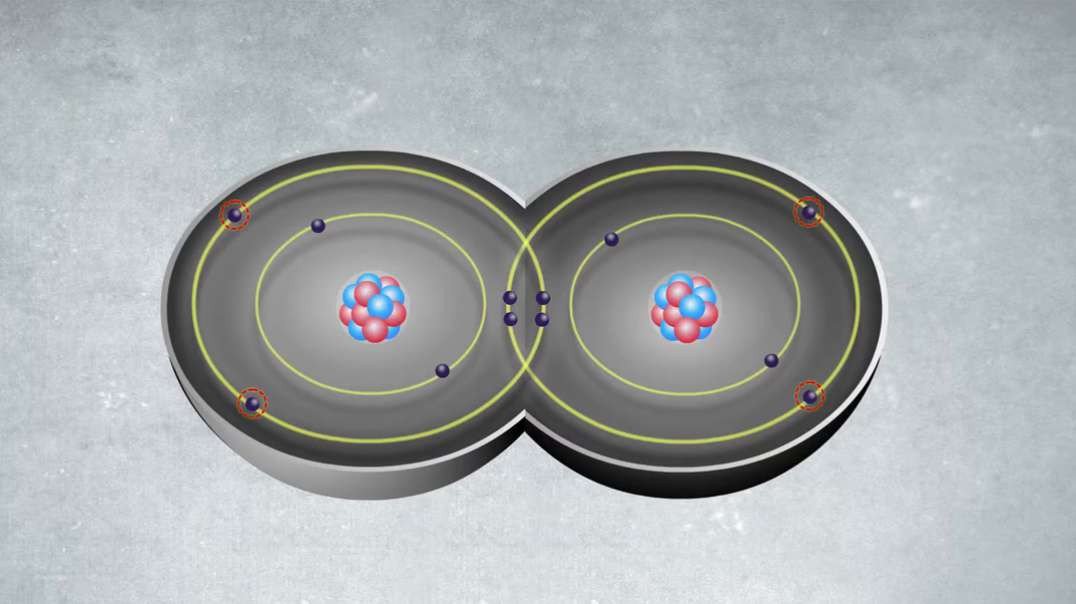
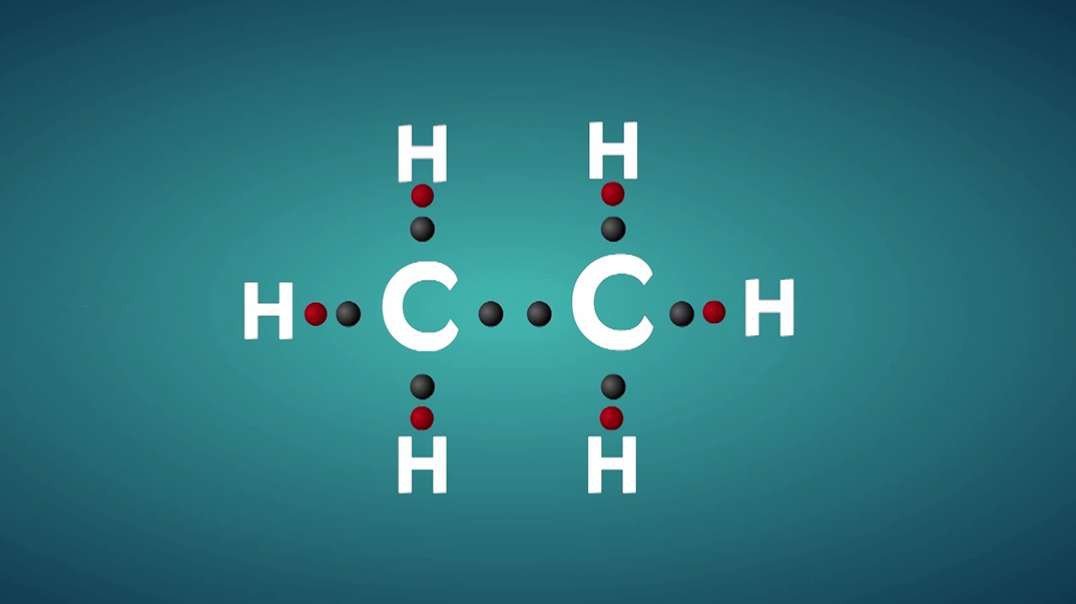
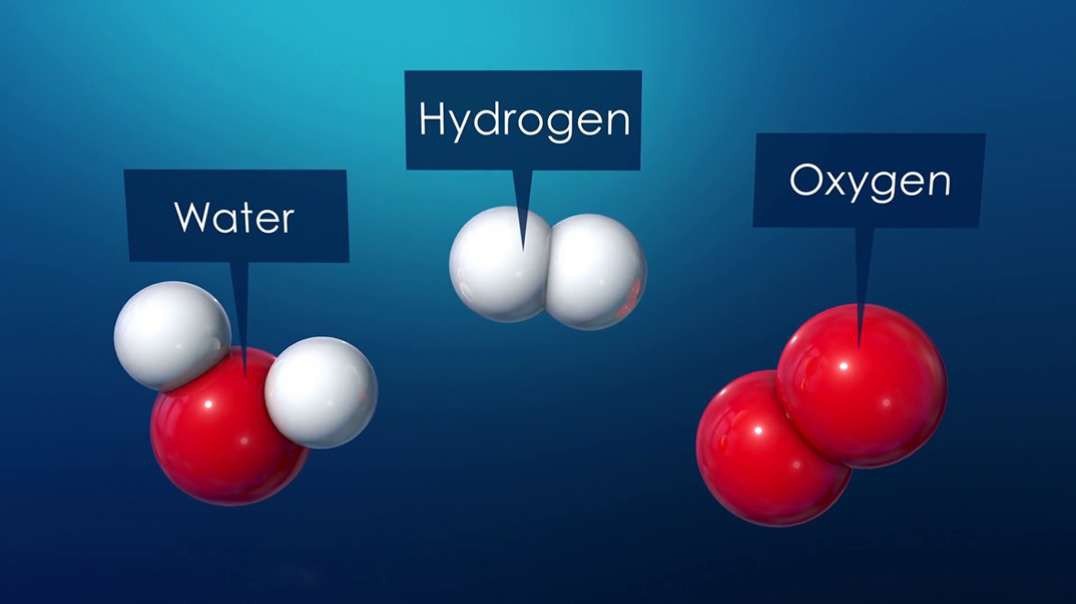
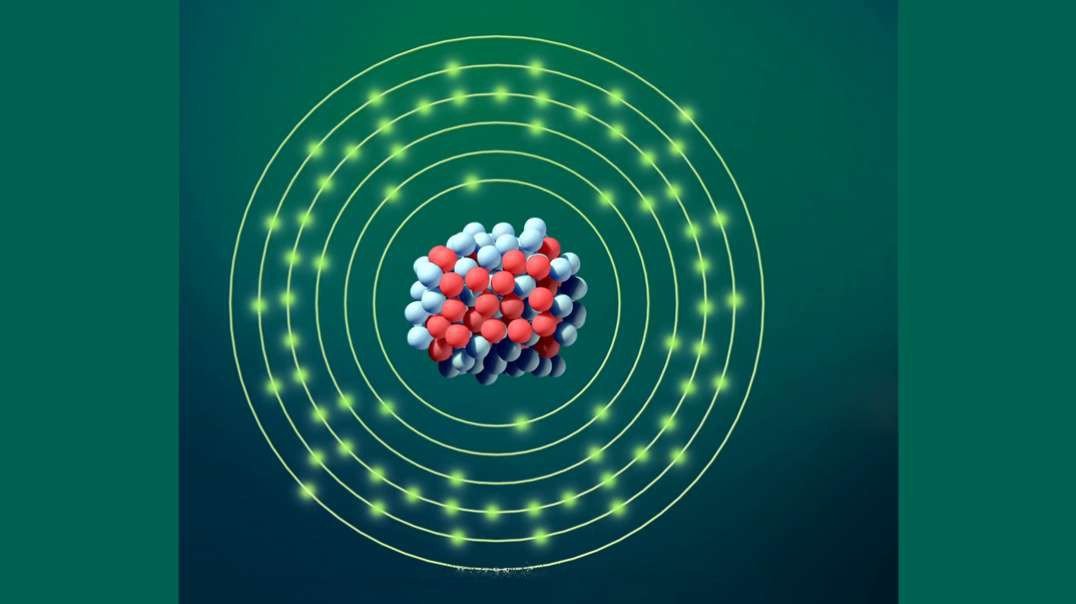
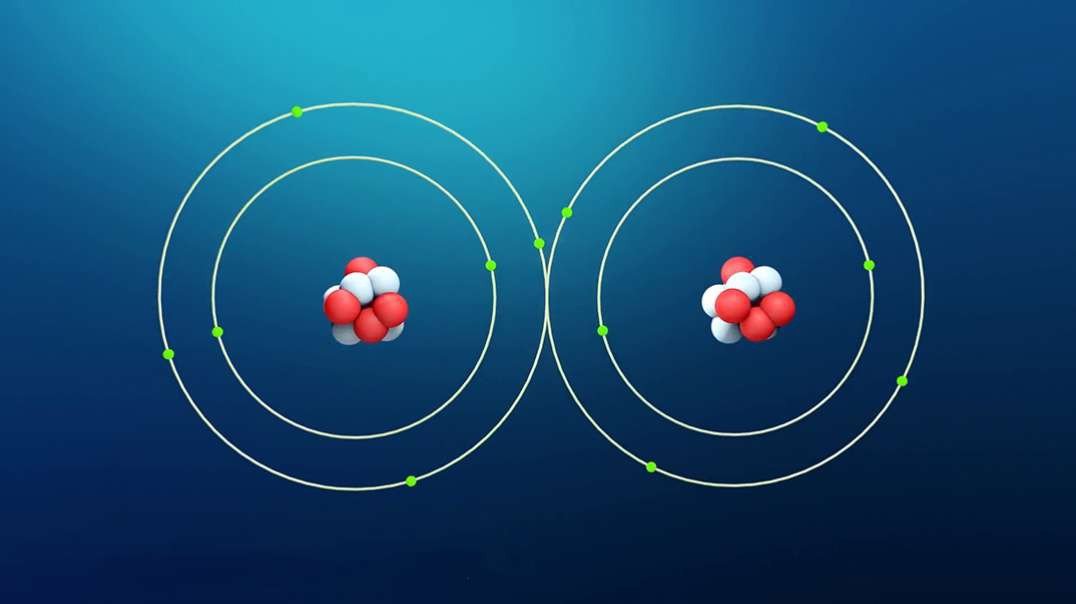
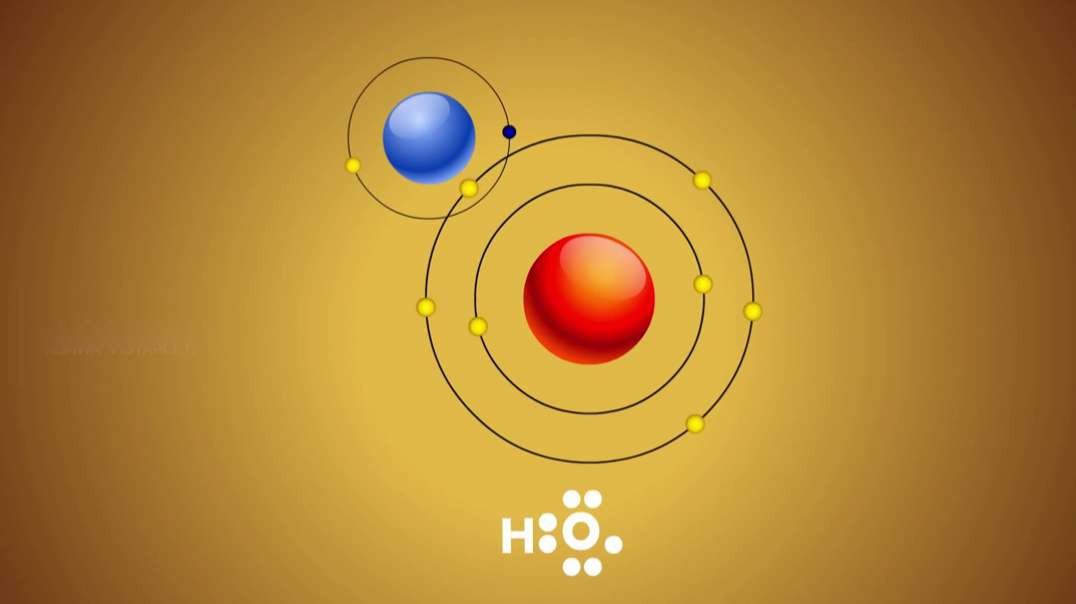
An element is a single type of atom, while a compound consists of two or more types of atoms.
Elements cannot be further divided into smaller units without using large amounts of energy. Compounds, meanwhile, can often have their bonds broken using reasonable amounts of energy, such as heat from a fire.
Examples of elements : gold, copper, carbon, and oxygen.
Example of compound :Table salt, i.e., sodium
chloride (NaCl), a compound that is composed of elements sodium (Na) and chlorine (Cl).
Show more
0 Comments
sort Sort By