:
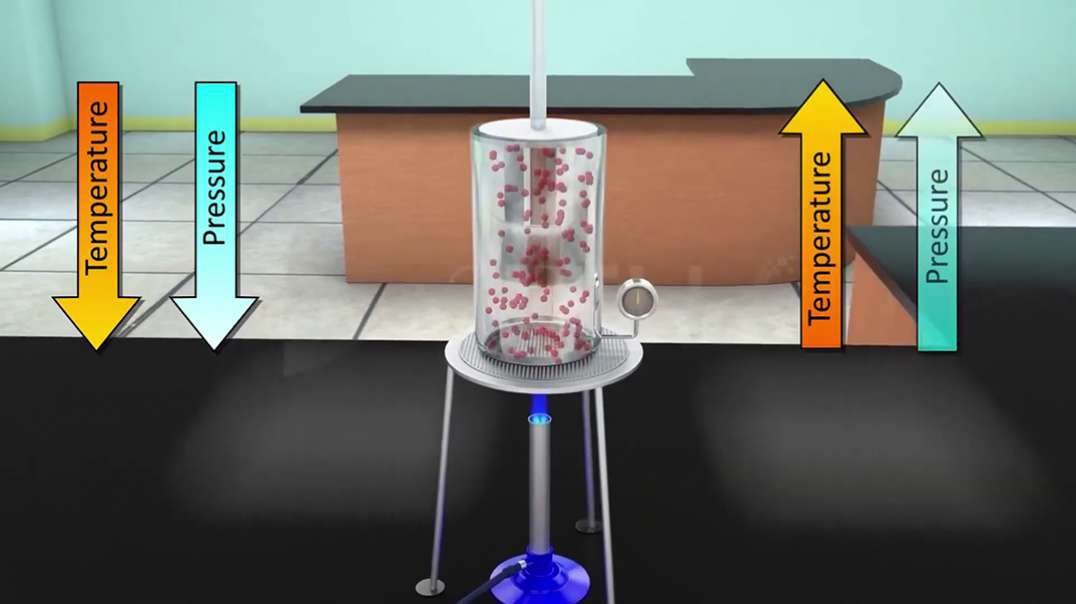
Gay-Lussac's law
Gay-Lussac's law states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas when the volume is kept constant. Mathematically, it can be written as: {\displaystyle {\frac {P}{T}}=k}. It is a special case of the ideal gas law.
What is Gay Lussac's law formula?
Gay-Lussac s Law states that the pressure of a given mass of gas varies directly with the Kelvin temperature when the volume remains constant. Gay-Lussac s Law is expressed in a formula form as P1/T1=P2/T2.
What is Gay Lussac's law explain with two examples?
Gay-Lussac's gas law is a special case of the ideal gas law where the volume of the gas is held constant. ... This example problem uses Gay-Lussac's law to find the pressure of a heated container. GAY-LUSSAC'S LAW EXAMPLE. A 20 L cylinder containing 6 atm of gas at 27 °C.
