:
Mixtures with Water
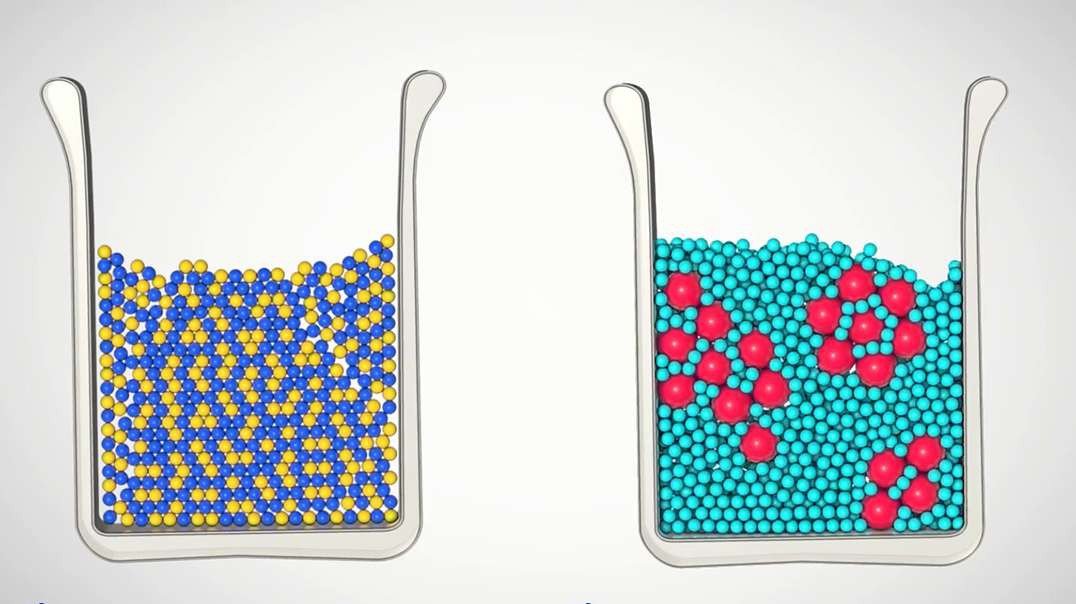
Today, we're going to talk about mixtures, specifically mixtures with water. A mixture is a physical, not a chemical, combination of two or more substances. Each substance in a mixture keeps its individual characteristics. A solution, also called a homogeneous mixture, consists of very tiny particles mixed so uniformly that the mixture has the same properties throughout. In contrast, a heterogeneous mixture consists of significantly larger particles that are not uniformly distributed and are more easily seen. The components of a heterogeneous mixture can usually be separated easily. Homogeneous water-based mixtures are called aqueous solutions. The dissolved substance is called the solute, and the substance that dissolves the solute, in this case water, is called the solvent. Water is sometimes referred to as the universal solvent because it can dissolve more substances than any other liquid. Water can dissolve many substances because of the polar nature of water molecules. This allows water molecules to surround and hold on to other small polar molecules. As a result, the polar molecules spread out evenly throughout the water. Remember, water is the solvent. Water's polar nature also allows the slightly positive and slightly negative charges on its molecules to dissolve ionic compounds known as salts. Water does this by separating the compound into negatively and positively charged particles called ions. The negative poles of water molecules surround the positive ion from the compound, and the positive poles of water molecules surround the negative ion from the compound. Solutes and solutions are so small and uniformly distributed that solutions are always transparent. You can see right through them. Now, let's talk about heterogeneous aqueous mixtures. There are two main types: colloids and suspensions. Colloids and suspensions differ primarily in the size of their particles within the water. In colloids, the particles mixed in the water are larger than the water molecules, but are still too small to see with the naked eye. As big as these particles are, they're still small enough that the random motion of the water molecules keep them mixed within the water. In contrast, suspensions contain even larger particles than those in colloids, but the particles are just small enough to be suspended in water when stirred or shaken. Over time, however, the particles in a suspension start to settle to the bottom of the container. Heterogeneous water mixtures such as colloids and suspensions are never transparent. They're always cloudy, hazy, or opaque, and you can't see through them. In this example, the colloid is milk, and the suspension is sand in water. To recap, mixtures are physical rather than chemical combinations of two or more substances, and each substance in the mixture keeps its individual characteristics. A mixture may be a solution which is a homogeneous mixture consisting of very tiny particles mixed so uniformly that the mixture has the same properties throughout, or a mixture may be heterogeneous with larger particles that are not uniformly distributed and are more easily seen. Aqueous solutions are homogeneous water-based mixtures consisting of tiny ions or molecules dissolved in water. A solute is the dissolved substance in a solution. A solvent is the substance that dissolves the solute. Water is called the universal solvent because it dissolves more substances than any other liquid. Aqueous colloids and suspensions are heterogeneous mixtures consisting of larger particles that are less uniformly distributed and more easily separated than the particles in aqueous solutions.


