:
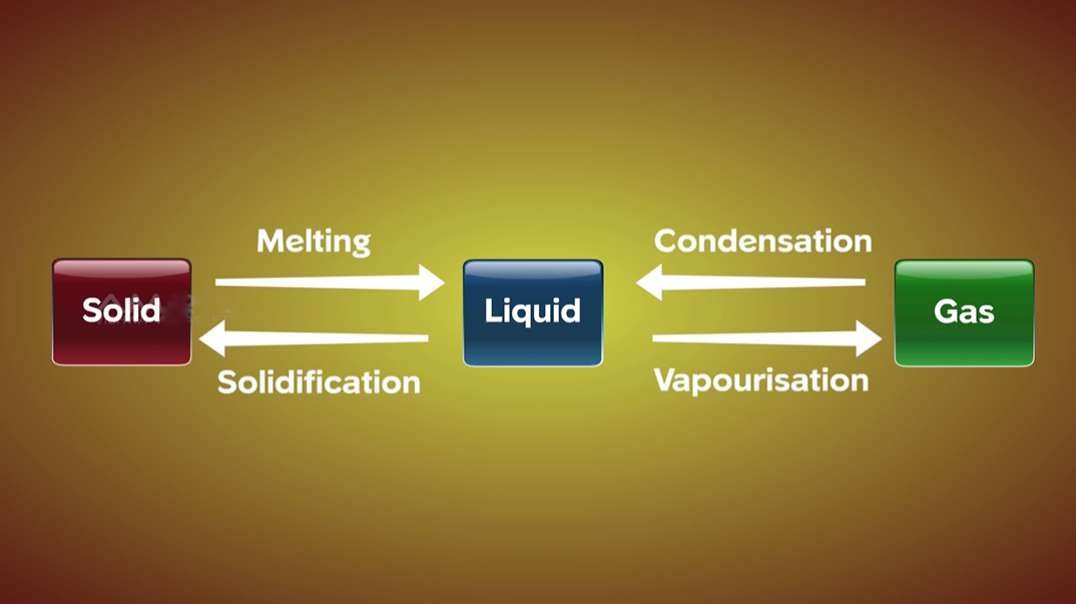
States of Matter: Ice, Water & Steam
This chemistry tutorial video explains the difference between ice, water and steam. It summarises the different properties of the 3 states of matter, then looks at the way the molecules in solids, liquids and gases are arranged, as well as how they move, to explain these properties.
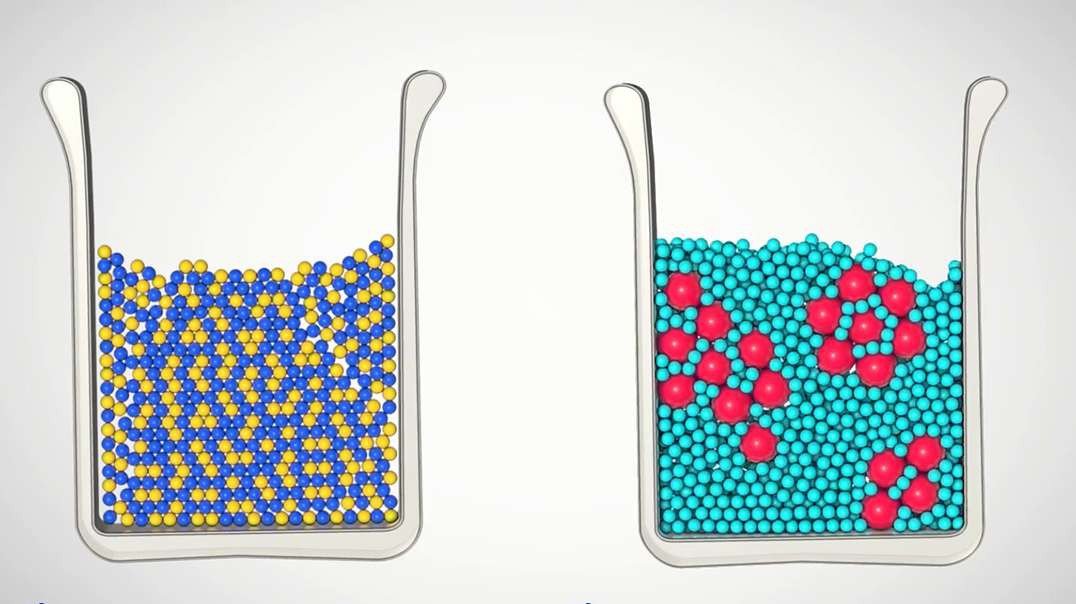
Animations of H2O molecules in ice, water and steam show how the molecules in solids are in a closely packed, fixed, regular pattern, with the molecules slowly vibrating backwards and forwards around a fixed position; molecules in liquids move randomly though faster, and vibrating, rotating and translating in a closely packed arrangement; whereas molecules in gases mostly translate with large distances between them, and moving randomly in straight lines unless they collide.
The relationship between temperature and molecular kinetic energy is introduced.