:
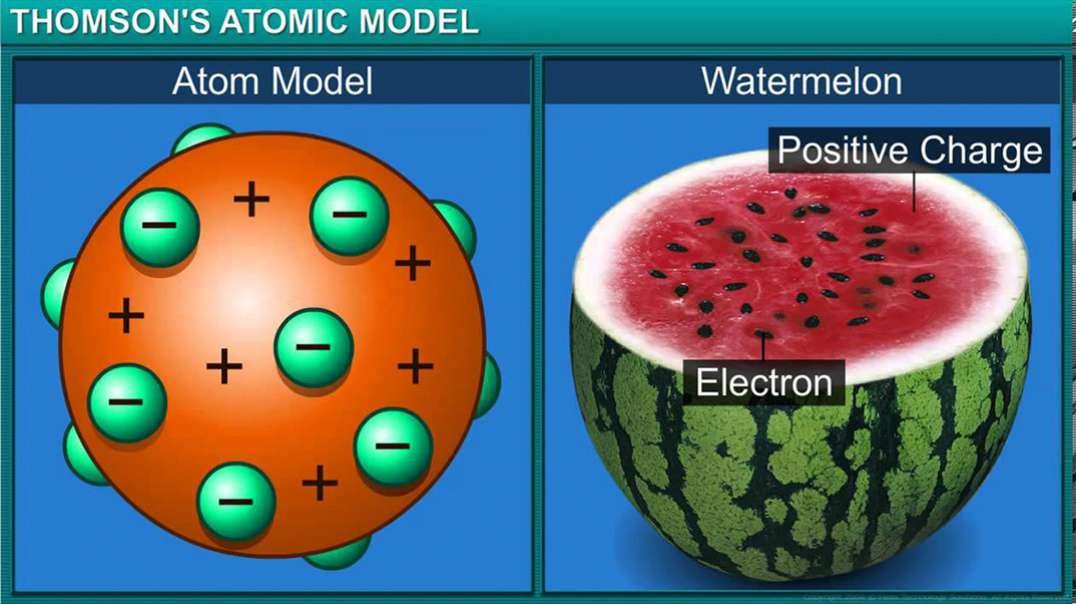
Rutherford's Model of An Atom: The gold Foil Experiment
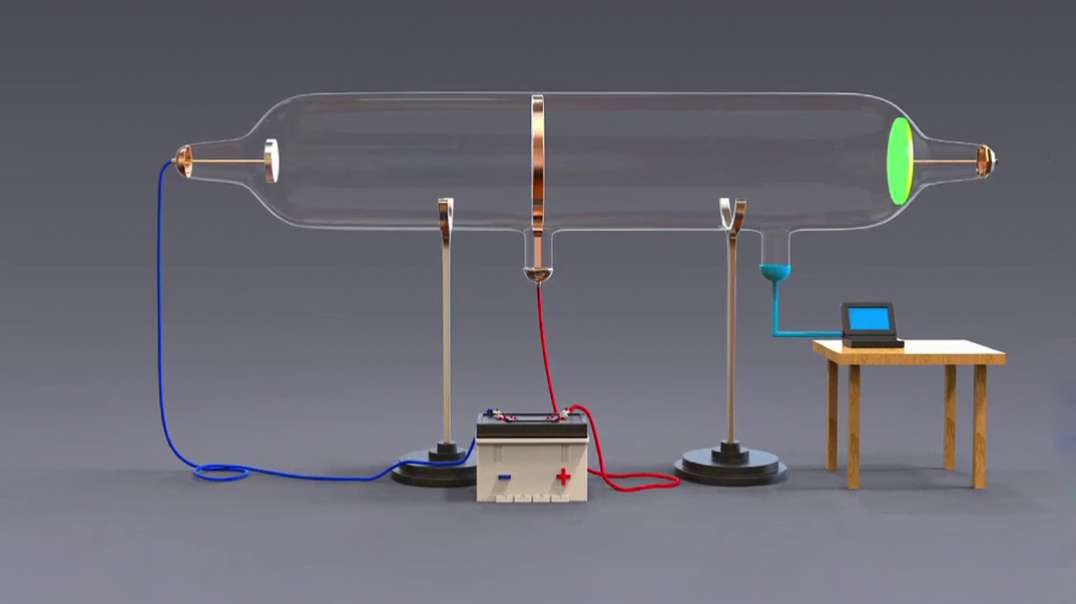
The Geiger-Marsden experiment, also called the gold foil experiment or the α-particle scattering experiments, refers to a series of early-20th-century experiments that gave physicists their first view of the structure of the atomic nucleus and the physics underlying the everyday world. It was first proposed by Nobel Prize-winning physicist Ernest Rutherford.
As familiar as terms like electron, proton and neutron are to us now, in the early 1900s, scientists had very little concept of the fundamental particles that made up atoms.
In fact, until 1897, scientists believed that atoms had no internal structure and believed that they were an indivisible unit of matter. Even the label "atom" gives this impression, given that it's derived from the Greek word "atomos," meaning "indivisible."
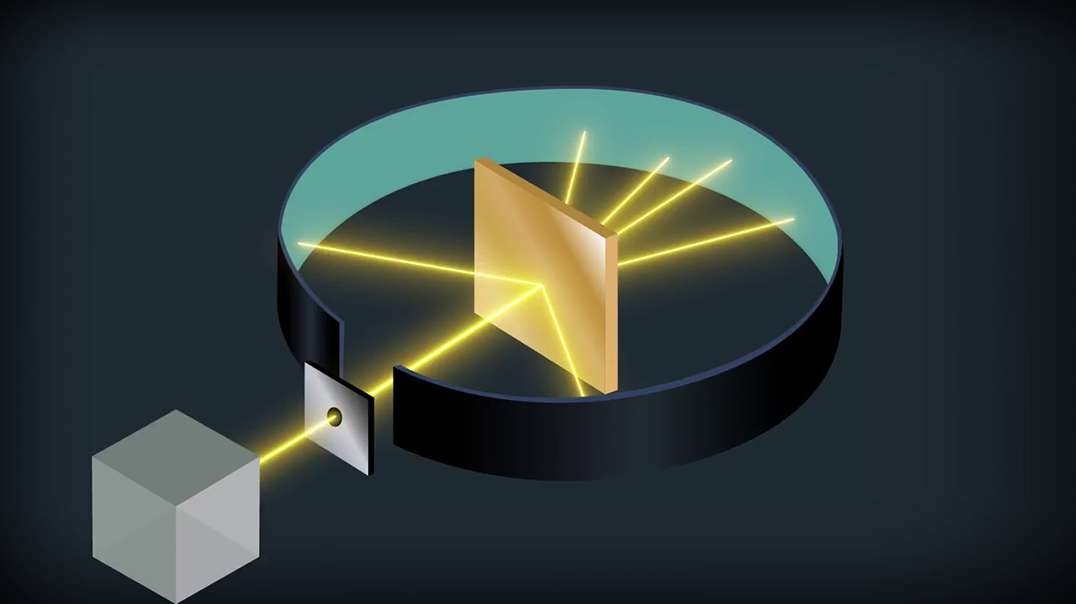
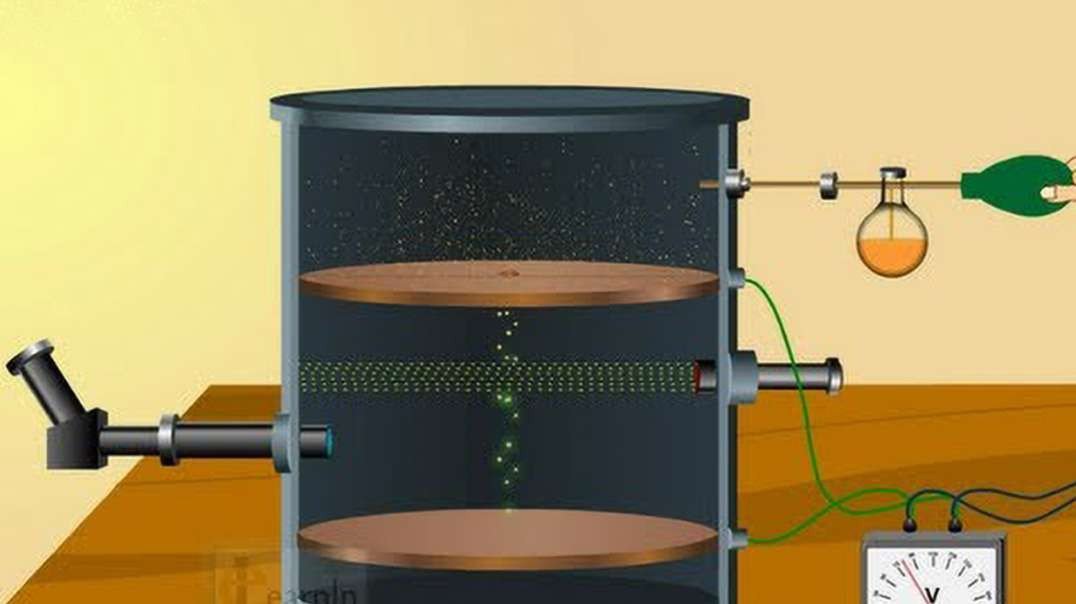
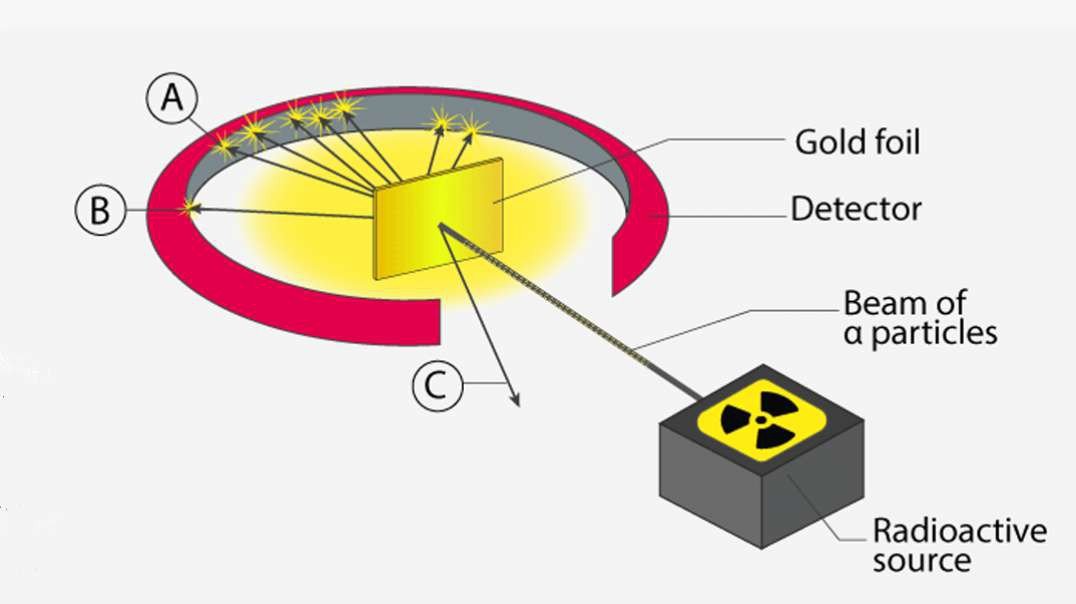
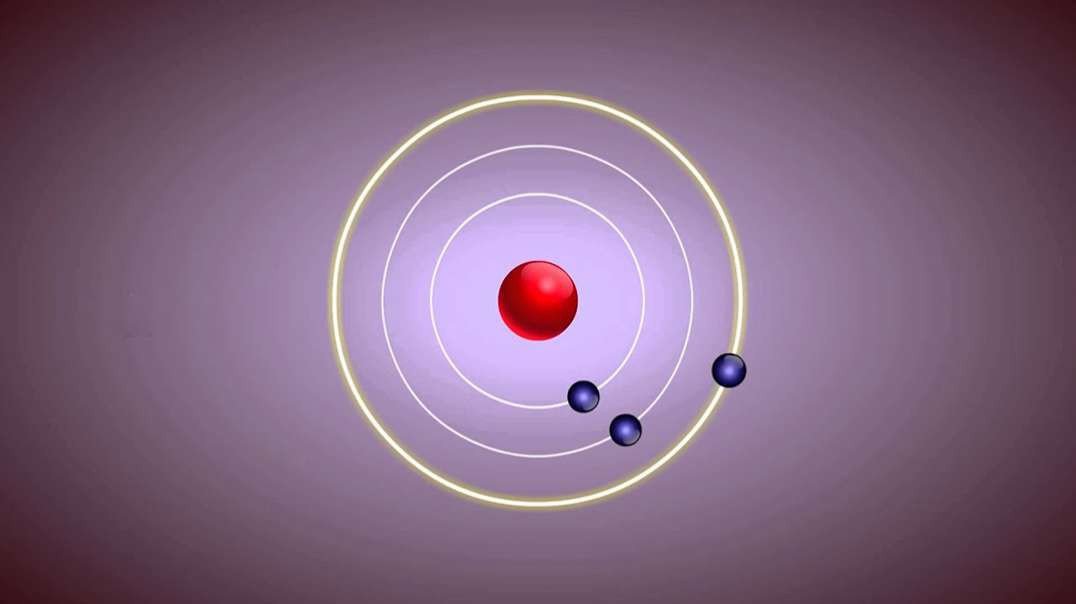
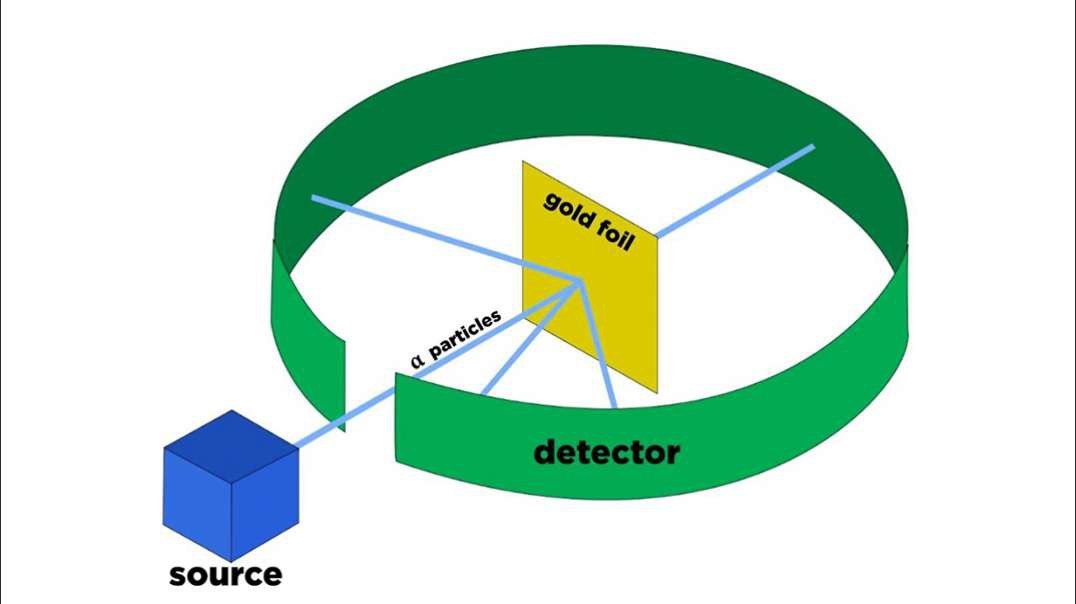
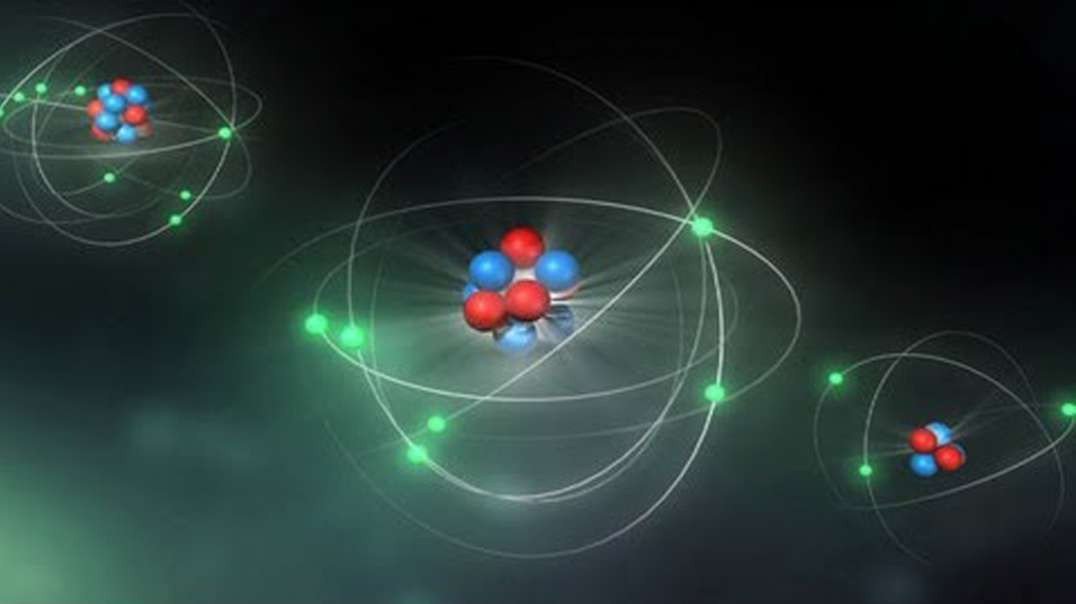
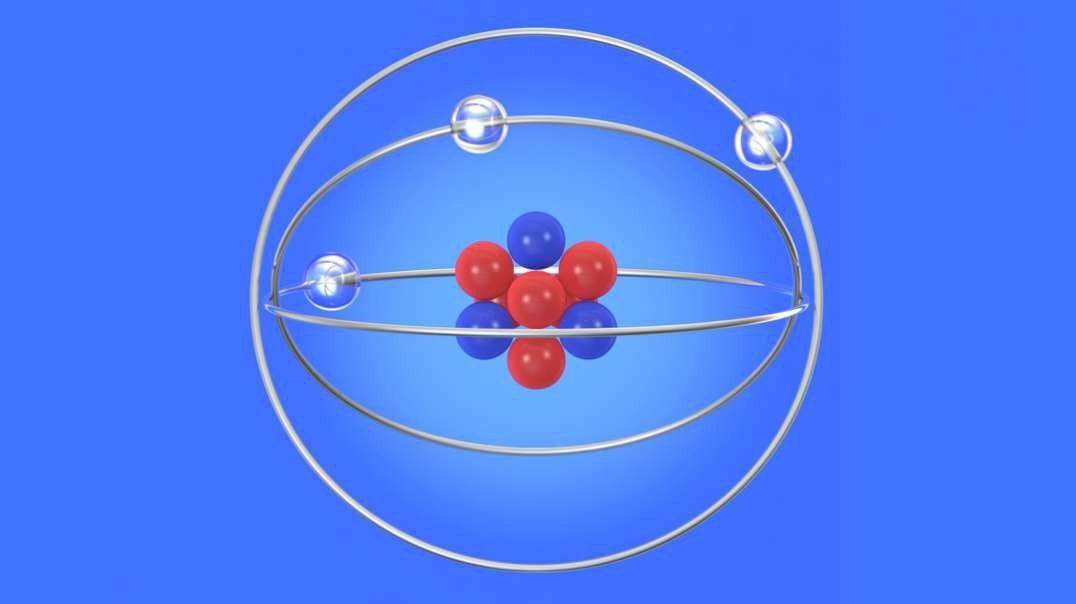
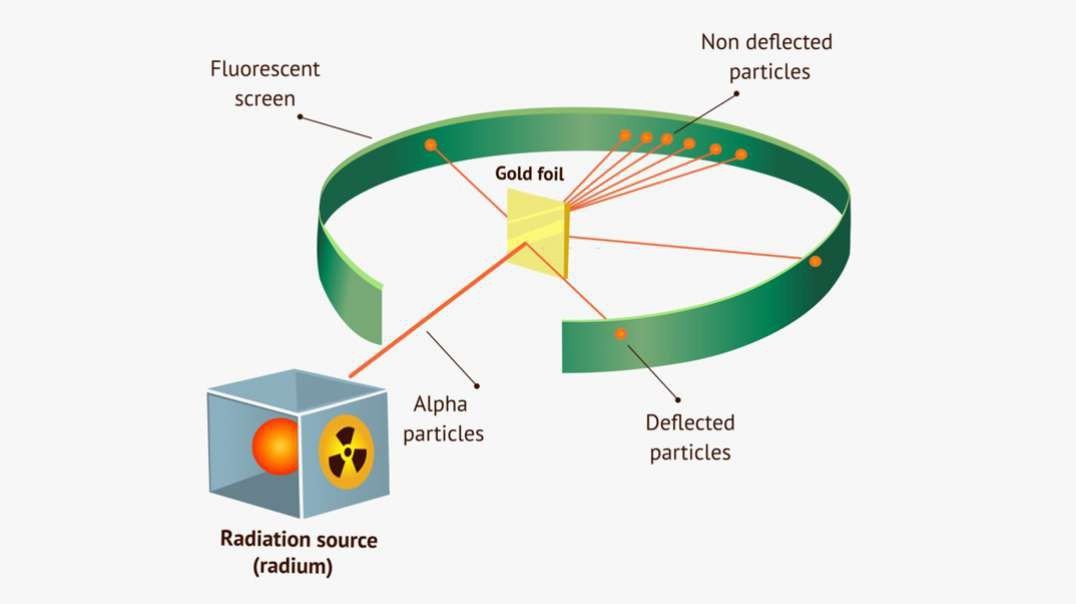
Rutherford performed an alpha particle scattering experiment on gold foil. Alpha particle source was kept in a lead block and a circular screen was placed around the foil. As alpha particles are scattered through the source and struck the zinc sulfide screen, these produce scintillations. Rutherford examined these scintillations in different portions on the screen and observed that most of the alpha particles penetrated through the foil showed no deflection. Rutherford concluded that most of the space in an atom is empty and at its center, there is a positively charged sphere called nucleus around which electrons are revolving in the atom.