:
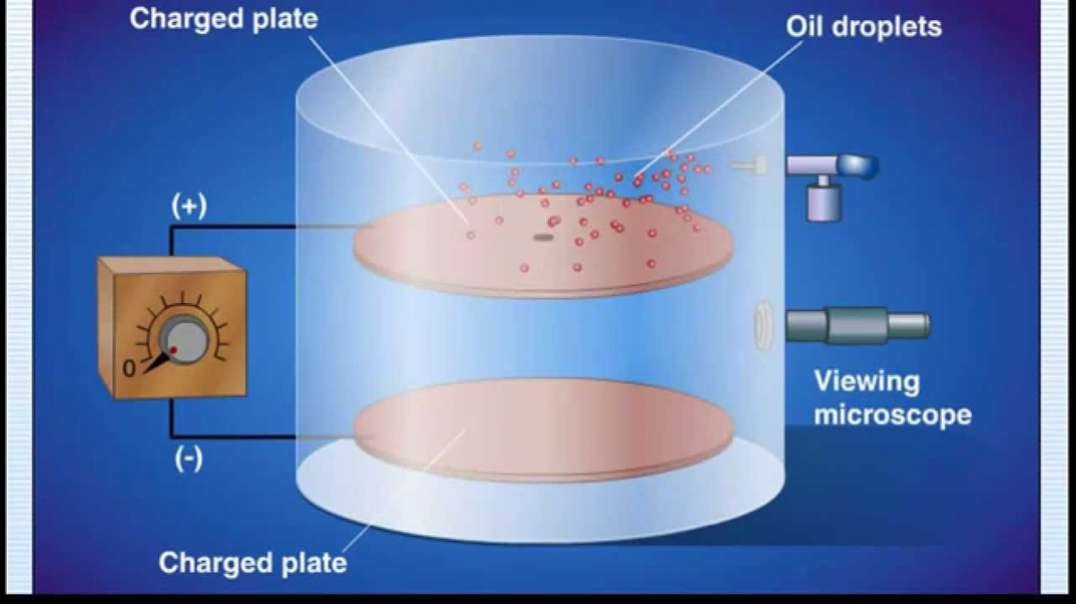
Discovery of the Charge of an Electron: Millikan Oil Drop Experiment:
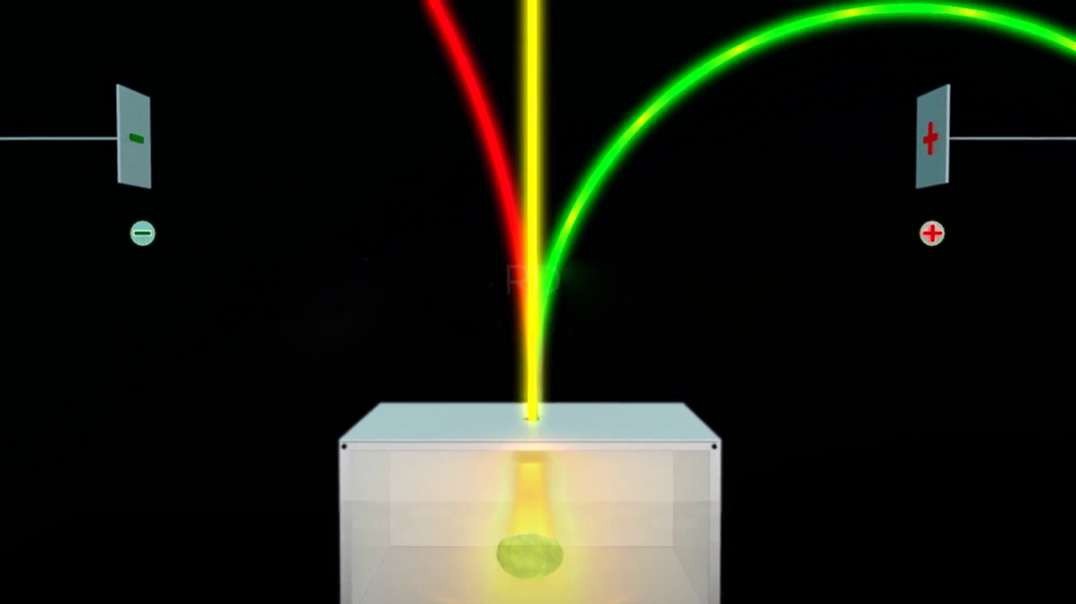
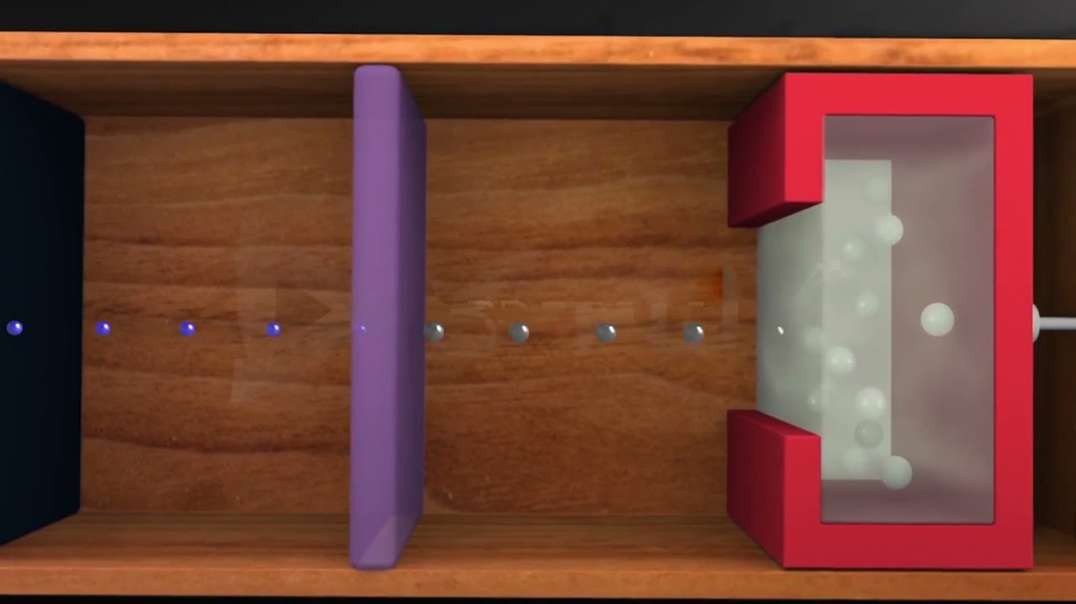
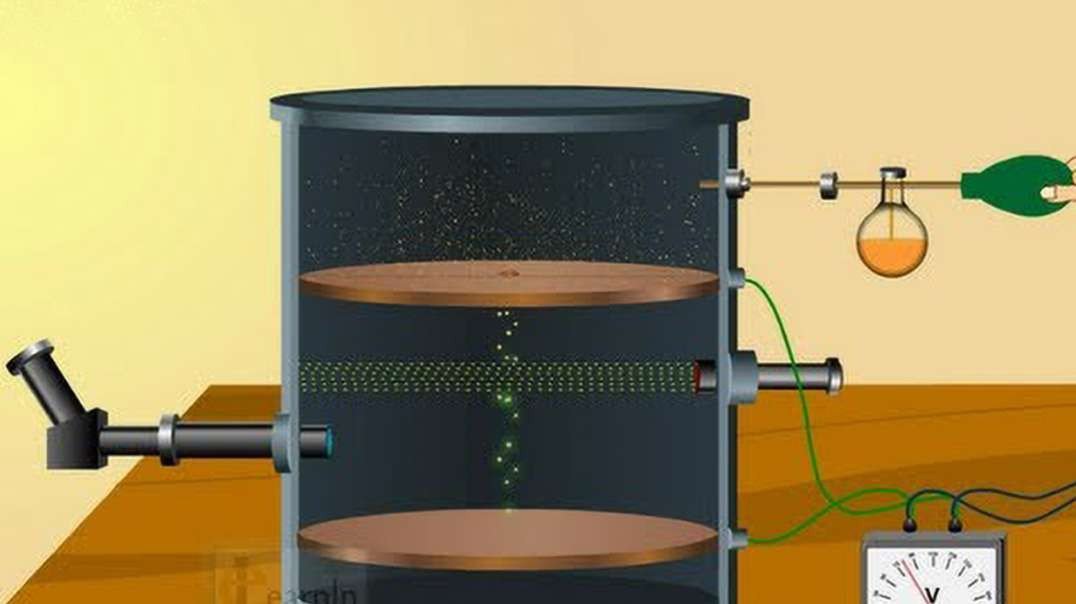
In 1909, Robert Millikan determined the charge of an electron (e–), using the oil drop
experiment. He found the charge of an electron to be 1.60 × 10–19 coulombs.
Combination of e–/m and e– values are used to determine the mass of an electron,
which is found to be 9.11 × 10–31 kg.
From Thomson’s experiment, e–/m = 1.76 × 108 C/g
From Millikan’s experiment, e– = 1.60 × 10–19 C
\ Mass of the electron = m =
/
e
e m
−
−
=
-19
8
1.60 × 10 C
1.76 × 10 C/g
= 9.11 × 10–28 g = 9.11 × 10–31 kg
From the above discussion, it follows that:
An electron is a fundamental particle of an atom carrying a negative charge and having
a very small mass. The mass of an electron is approximately 11837 times the mass
of a hydrogen atom.