:
Discovery of Cathode rays
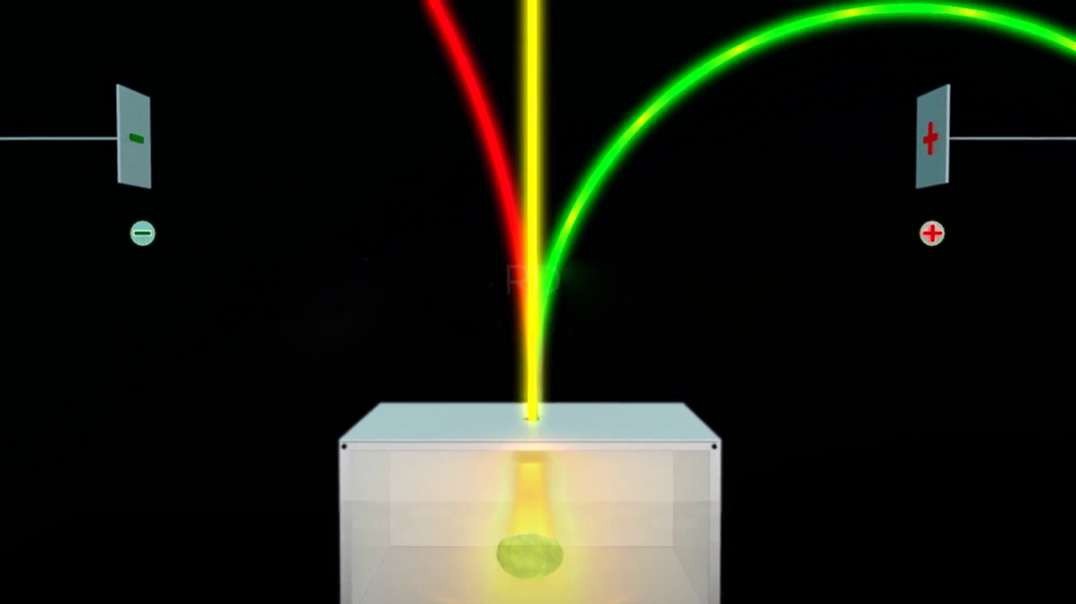
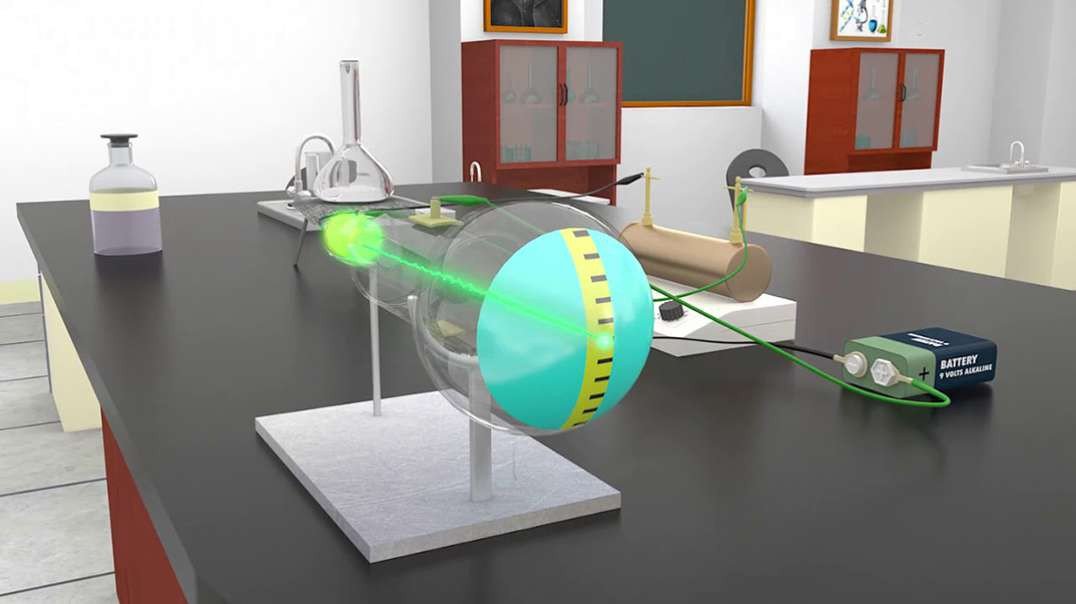
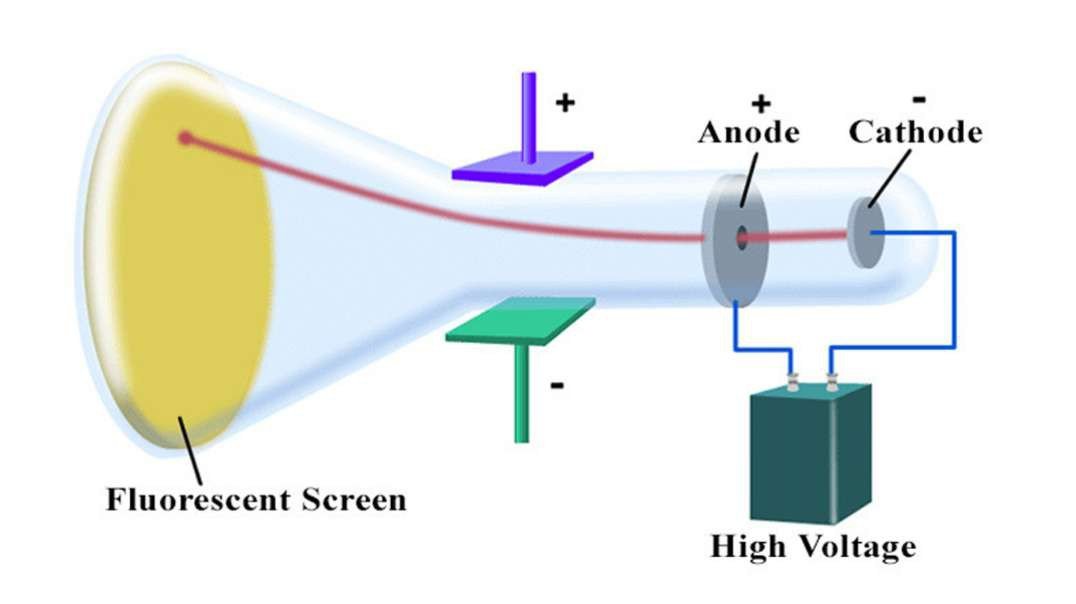
In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the electron. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen.
Who first discovered cathode rays?
Julius Plücker
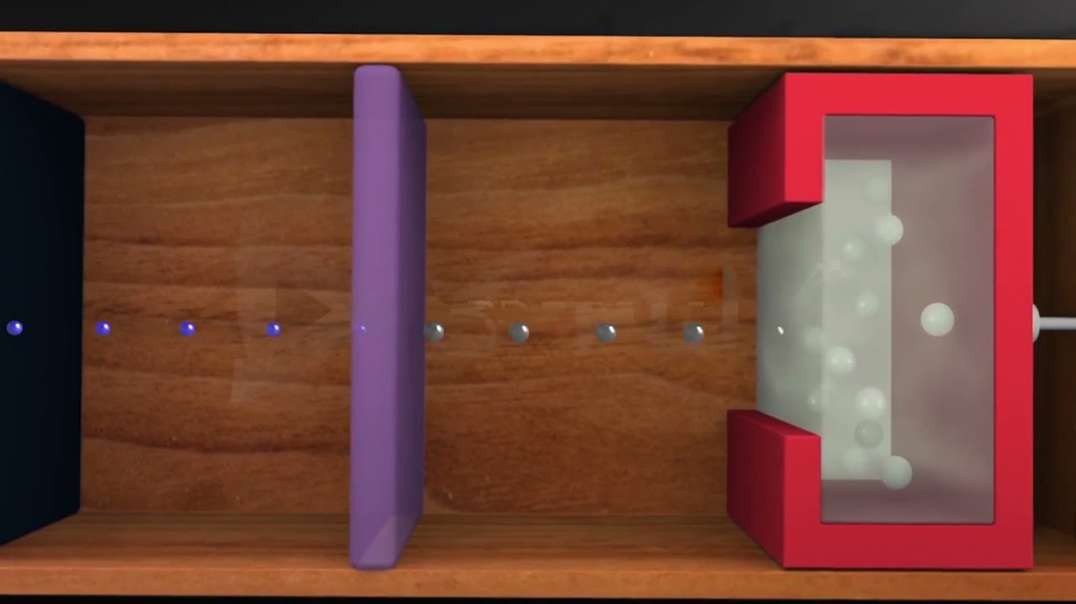
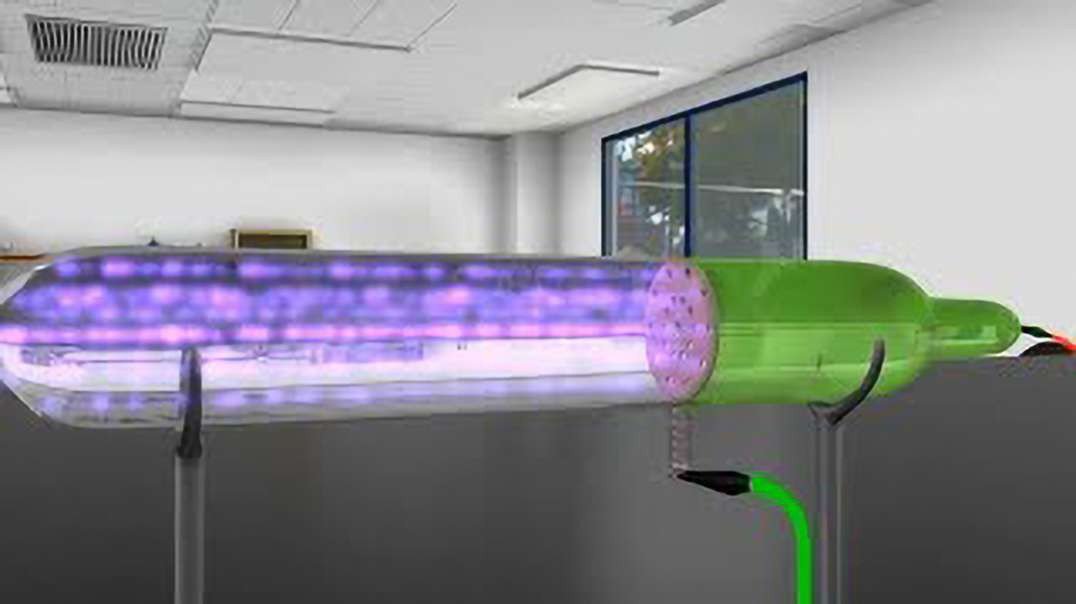
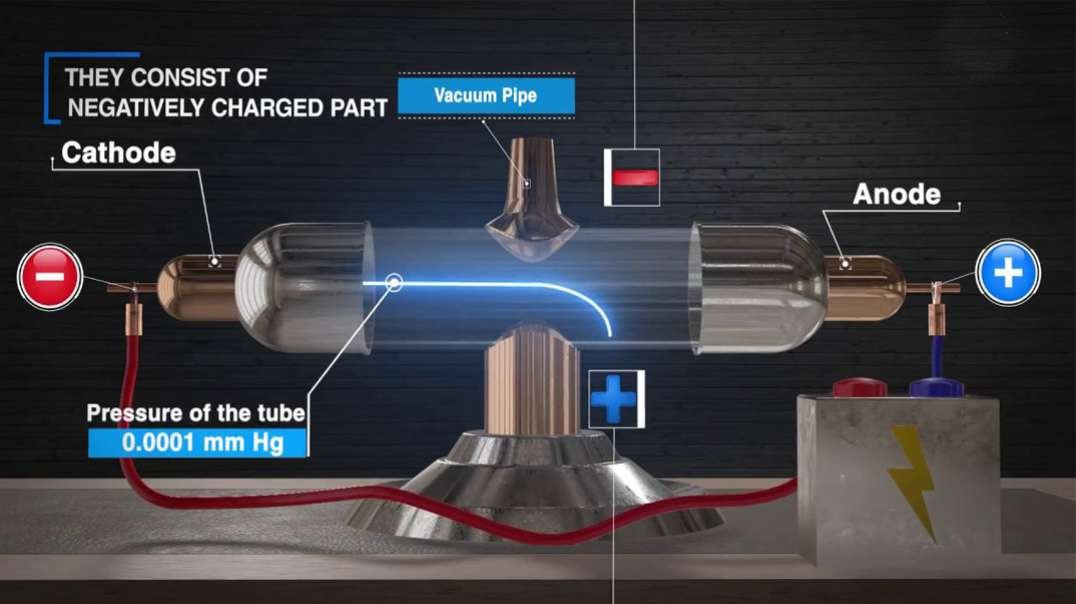
Cathode-ray studies began in 1854 when Heinrich Geissler, a glassblower and technical assistant to German physicist Julius Plücker, improved the vacuum tube. Plücker discovered cathode rays in 1858 by sealing two electrodes inside the tube, evacuating the air, and forcing electric current between the electrodes.
What is a an electron?
An electron is a negatively charged subatomic particle. It can be either free (not attached to any atom), or bound to the nucleus of an atom. Electrons in atoms exist in spherical shells of various radii, representing energy levels. ... The charge on a single electron is considered as the unit electrical charge.
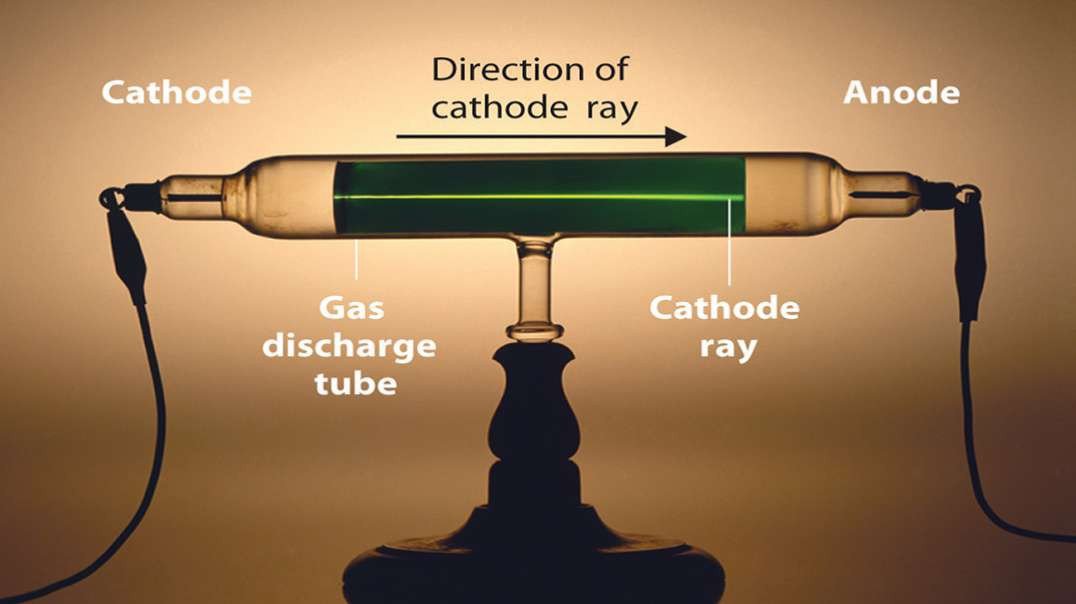
Cathode rays are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode.
How was cathode rays discovered?
Thomson's experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged "soup."