:
Discovery of the Mass of an Electron: Charge to mass ratio of an electron
The history of the atomic structure and quantum mechanics dates back to the times of Democritus, the man who first proposed that matter is composed of atoms. These theories could not gain much importance due to the lack of technology. The experiments conducted during the nineteenth century and early twentieth century revealed that even an atom is not the ultimate particle. The continued efforts of the scientists led to the discovery of subatomic particles like electrons, protons, and neutrons.
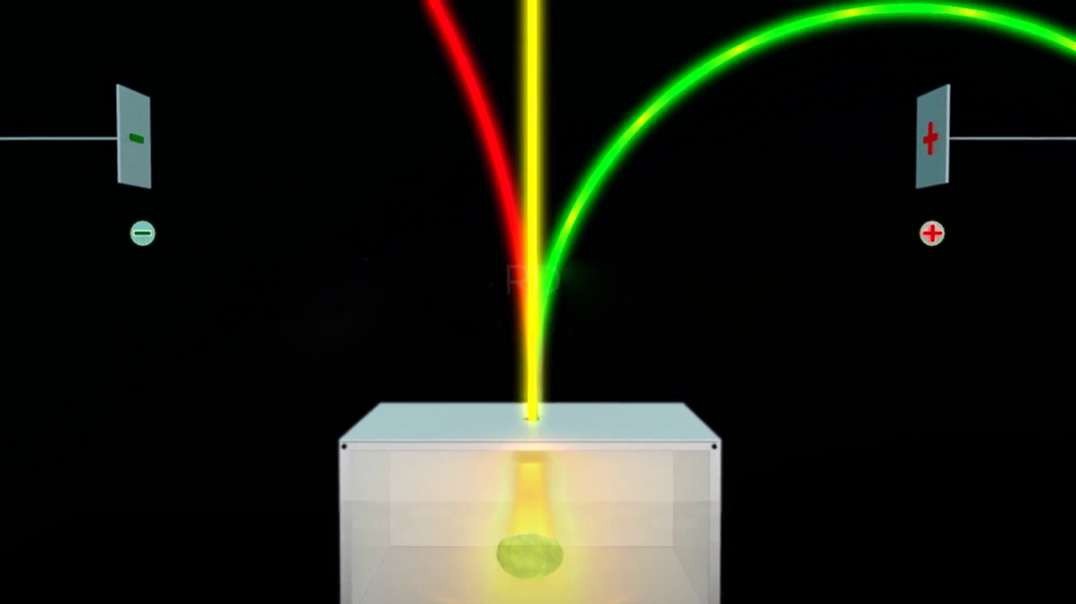
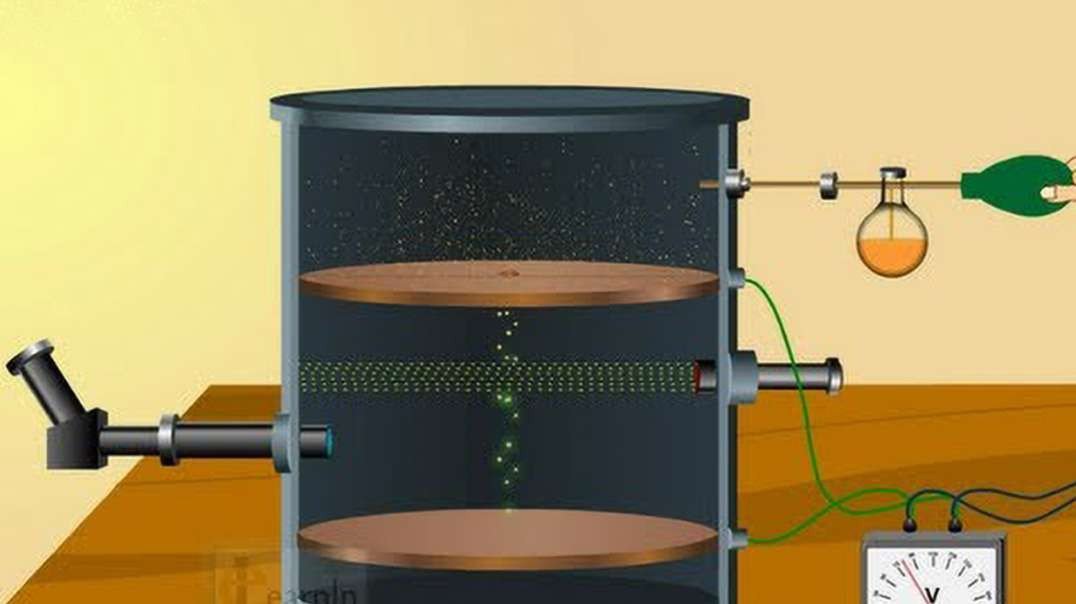
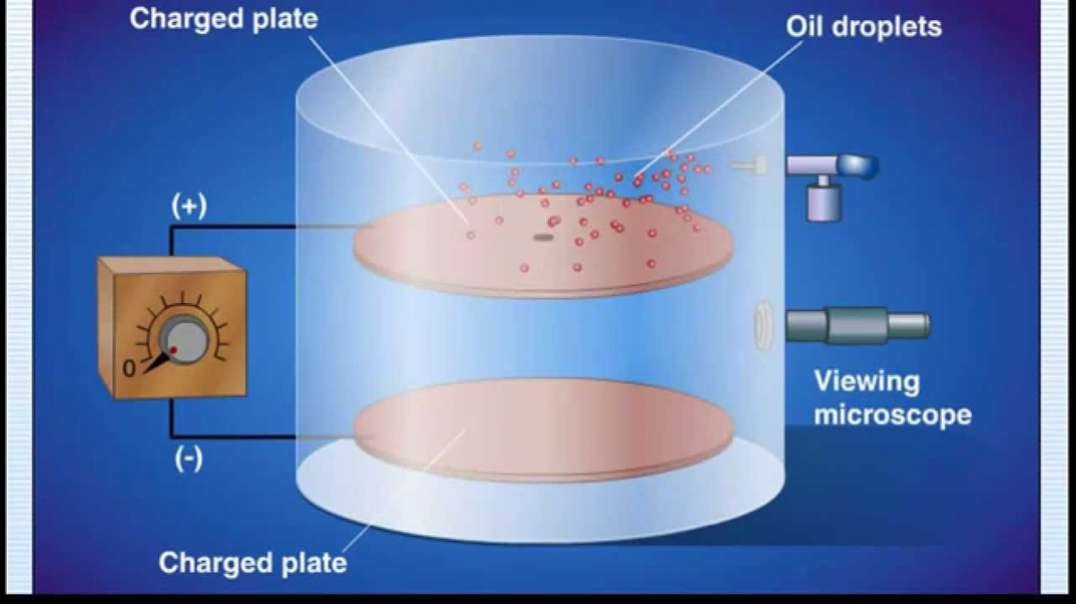
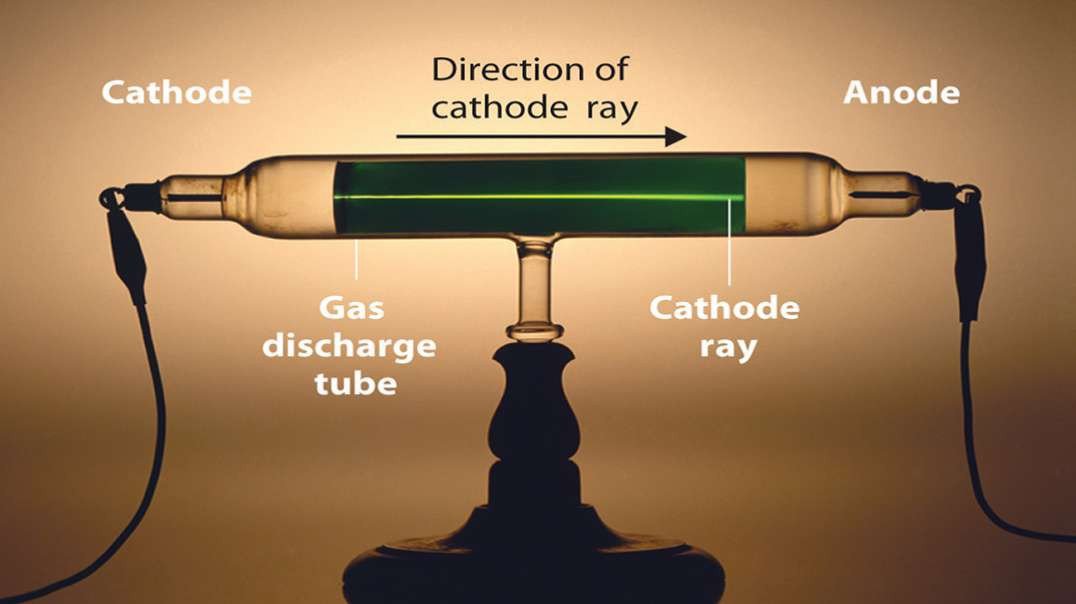
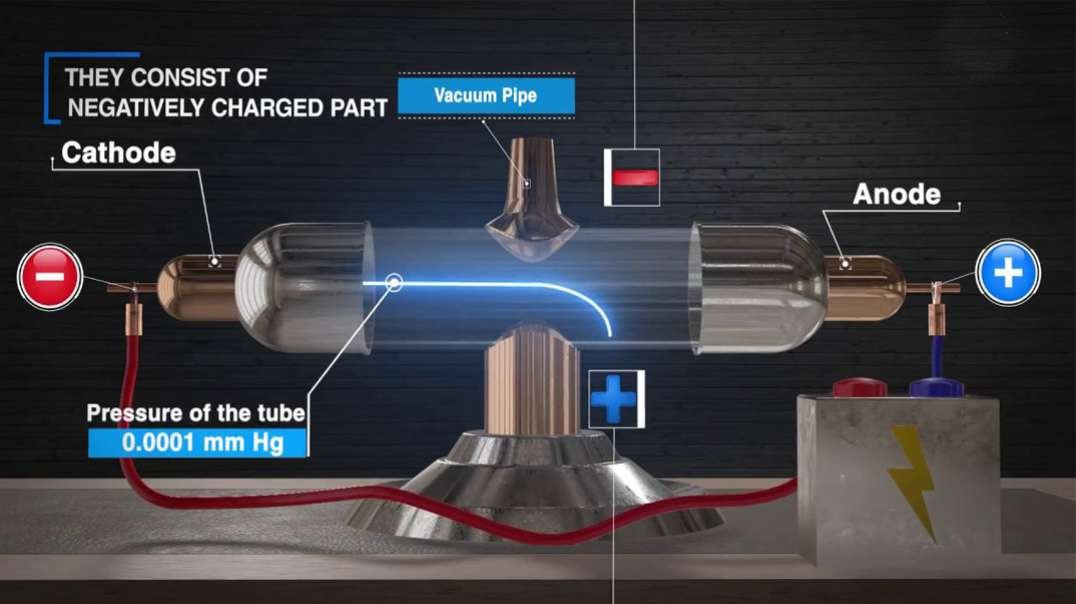
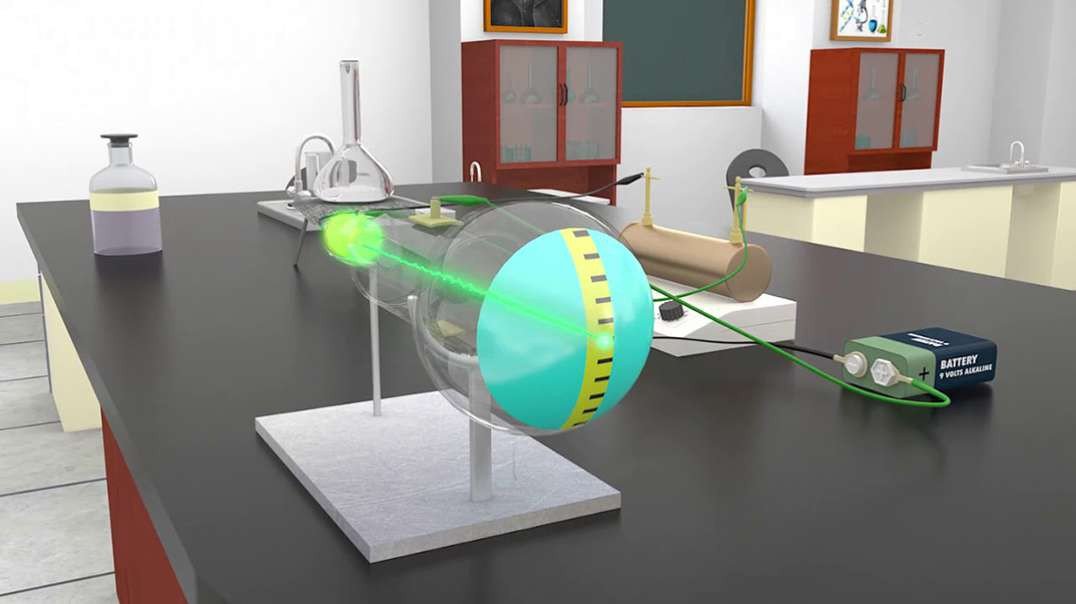
In the nineteenth century, J.J Thomson proposed Thomson’s Atomic Model discovered the electron to mark inception to the world of subatomic particles. Once the electron was discovered, he continued his experiments to calculate the charge and the mass of the electron. With the help of his experiments, he derived a formula for the calculation of charge to mass ratio of the electron.
Charge to Mass Ratio of Electron
The charge to mass ratio of the electron is given by :
e/m = 1.758820 × 1011 C/kg
Where,
m = mass of an electron in kg = 9.10938356 × 10-31 kilograms.
e = magnitude of the charge of an electron in coulombs = 1.602 x 10-19 coulombs.