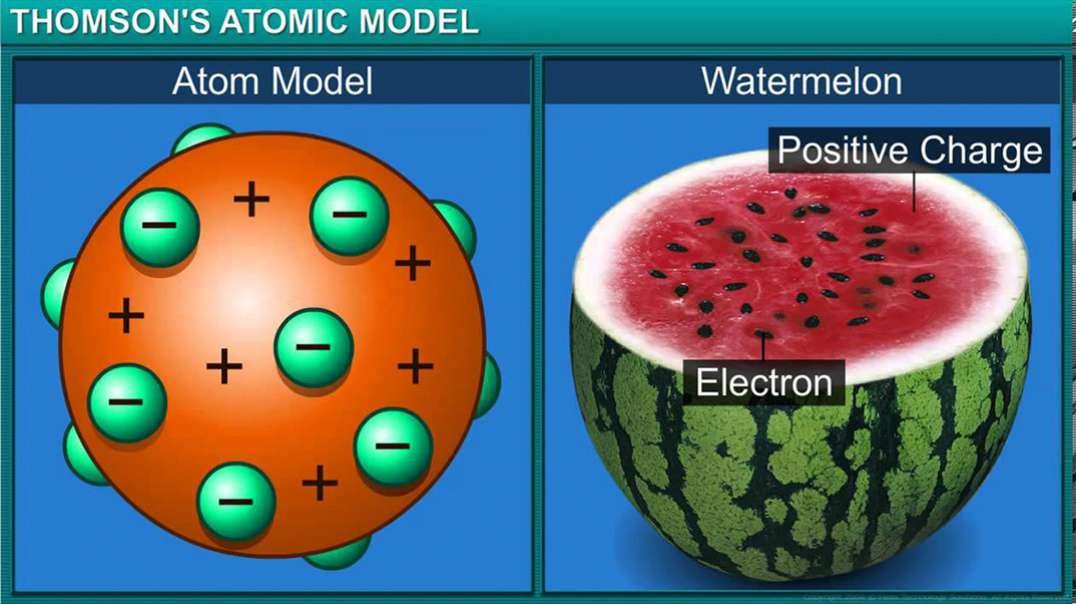
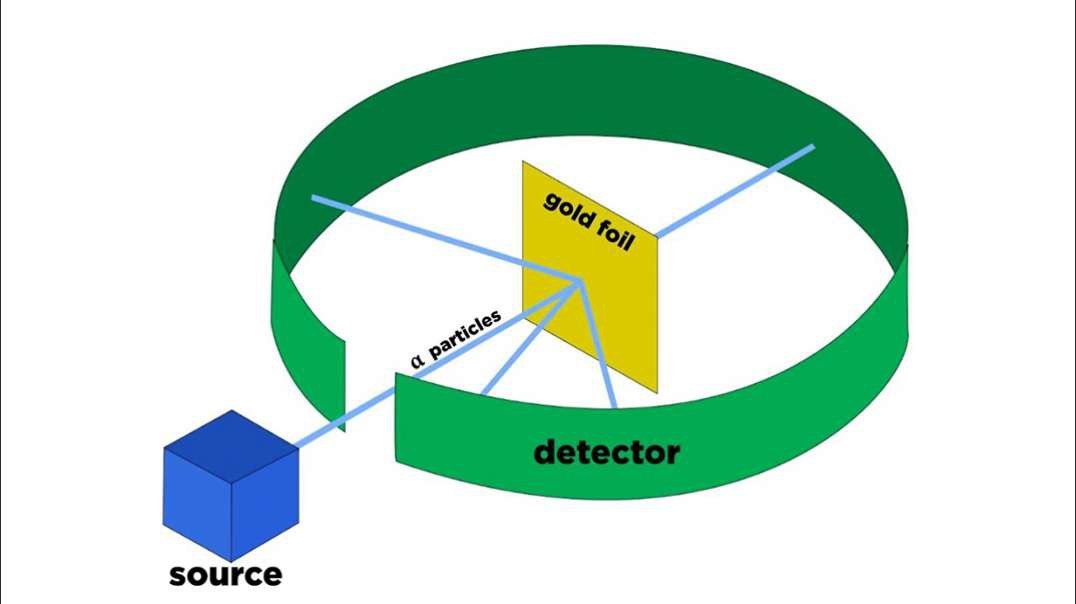
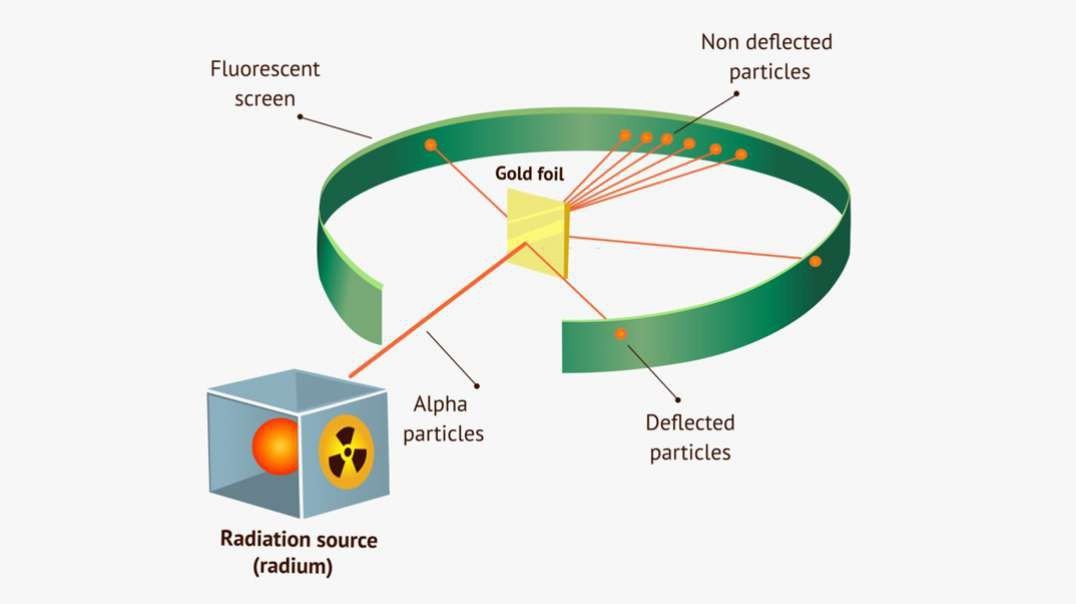
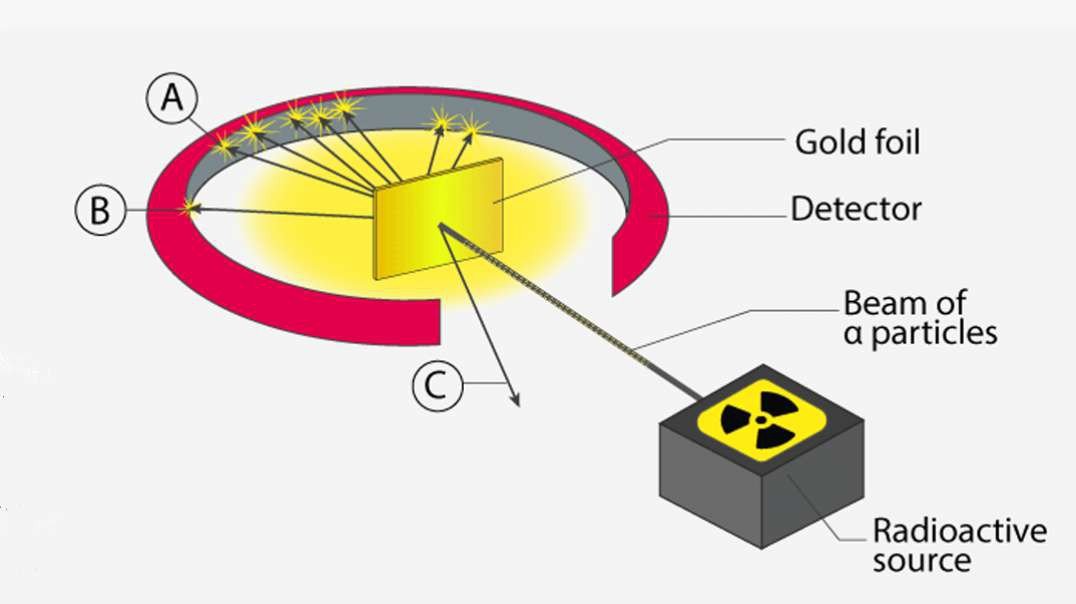
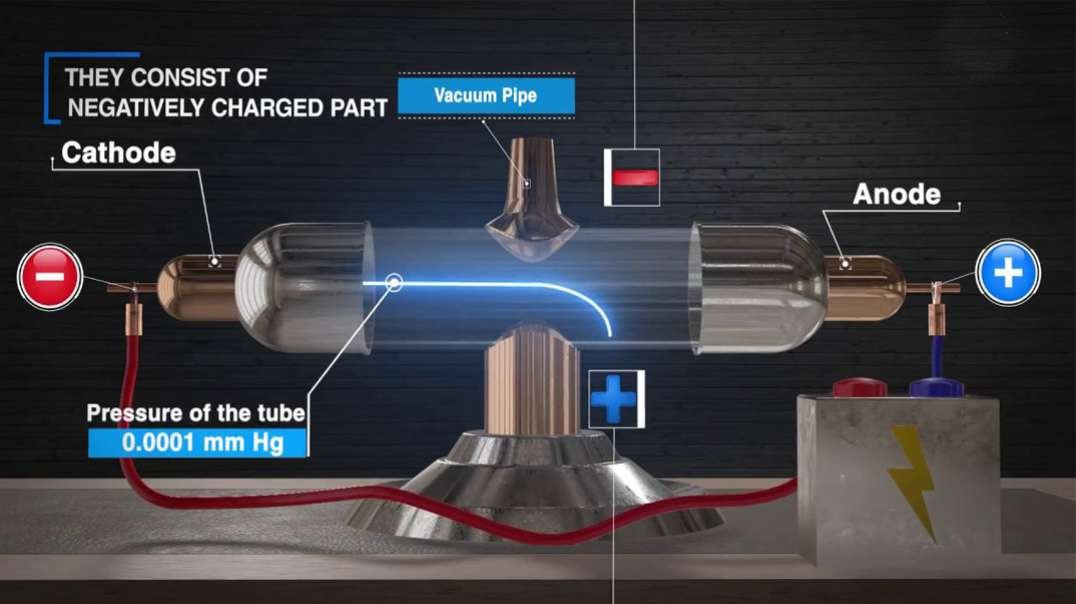
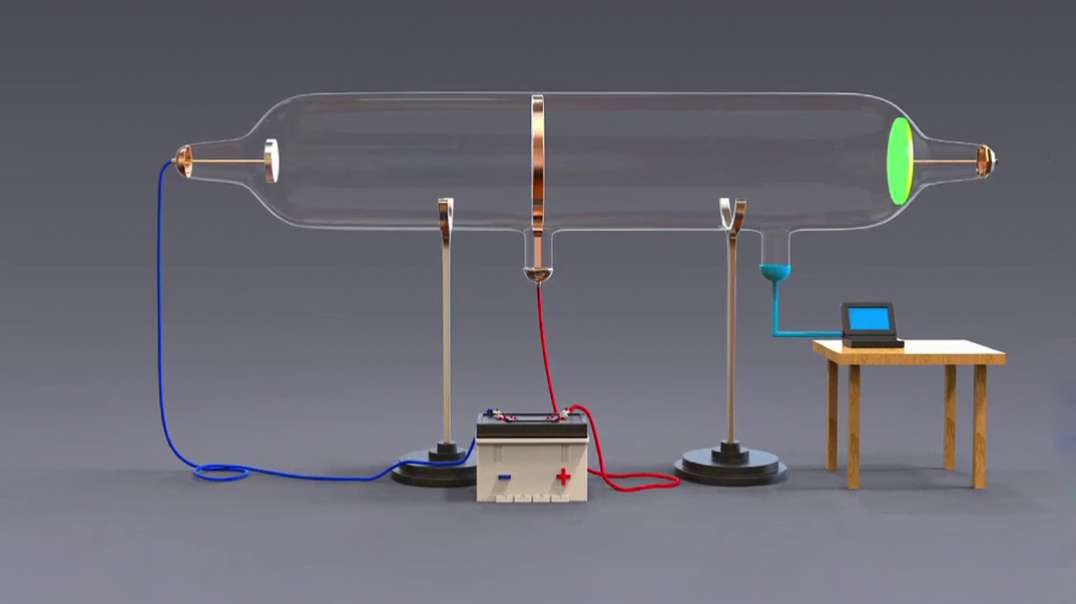
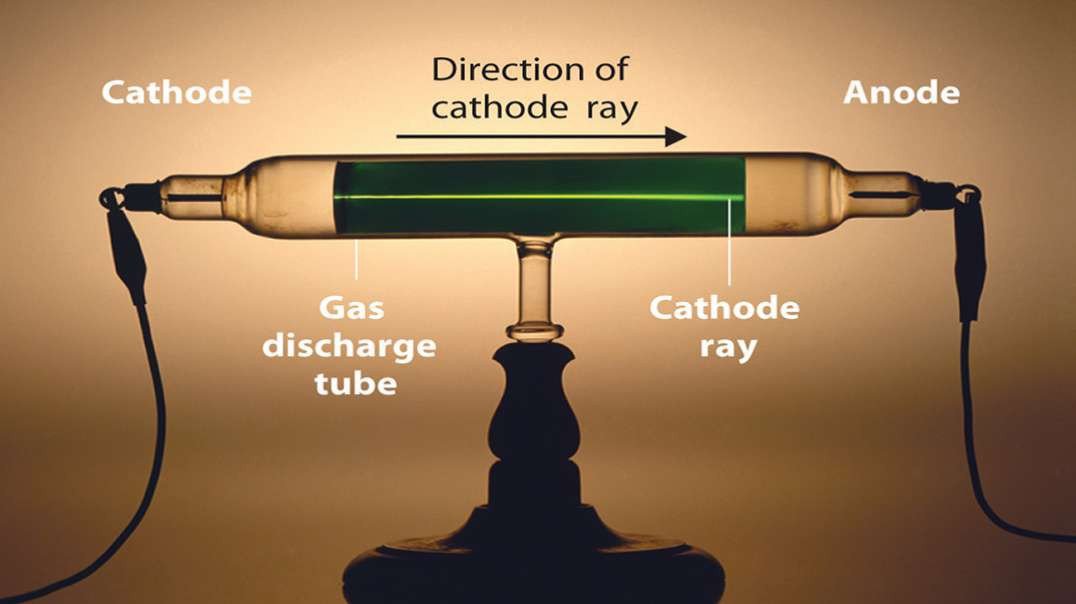
:
Avogadro's law
What is Avogadro's law class 11?
Avogadro's law, a statement that under the same conditions of temperature and pressure, equal volumes of different gases contain an equal number of molecules.
How do you explain Avogadro's law?
Avogadro's law states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules." For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
What is Avogadro's law example?
Avogadro's Law is in evidence whenever you blow up a balloon. The volume of the balloon increases as you add moles of gas to the balloon by blowing it up. If the container holding the gas is rigid rather than flexible, pressure can be substituted for volume in Avogadro's Law.