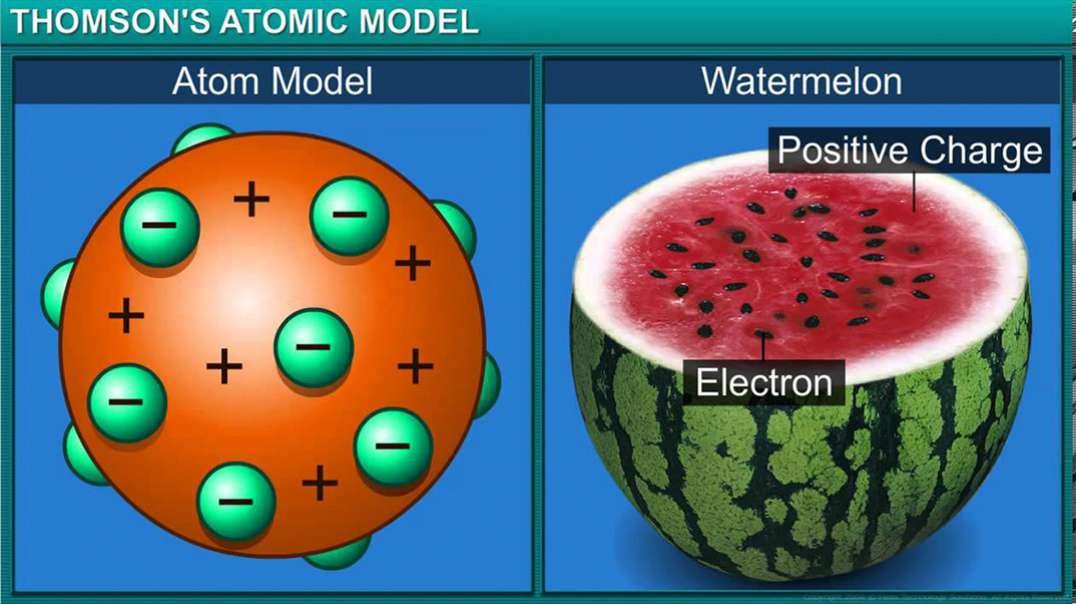
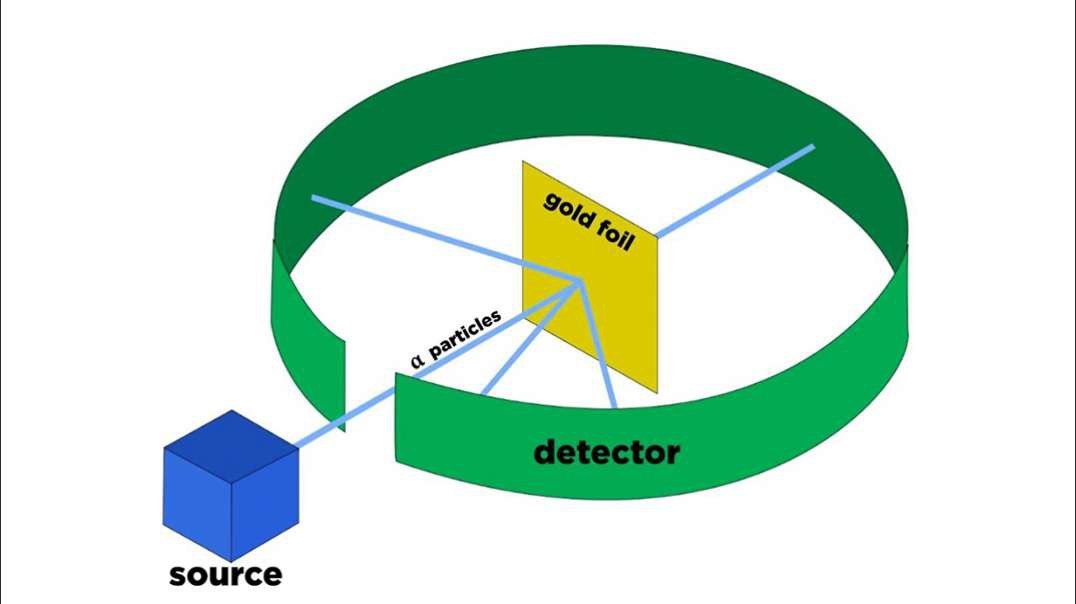
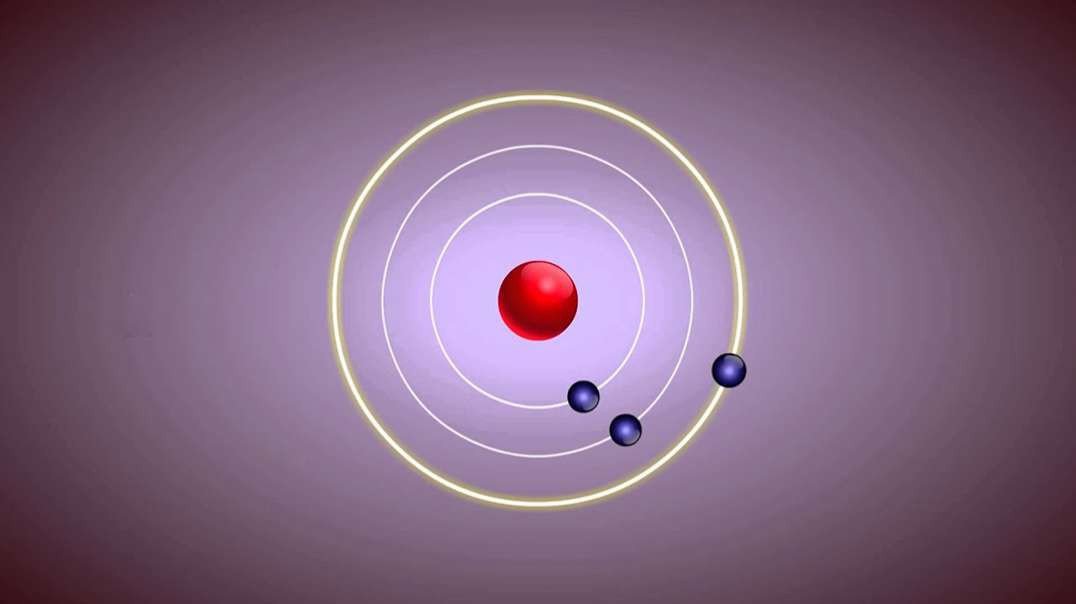
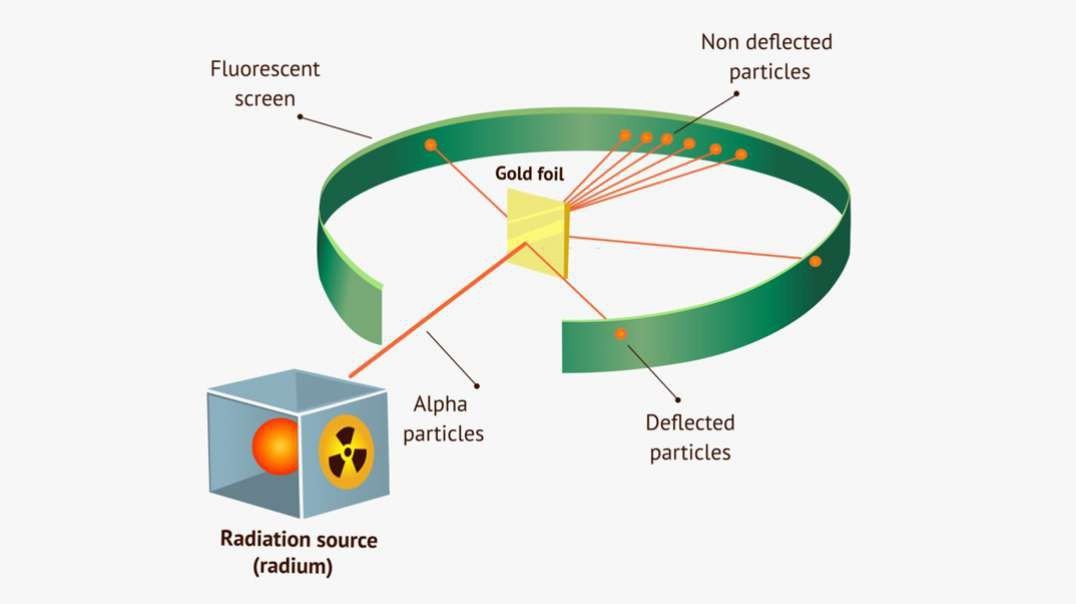
:
Hall Heroult's Process: Extraction of Pure Aluminium
0
0
2 Views
0 Purchases
Published on 17 Aug 2022 / In
Chemistry / Chemistry Grade 10
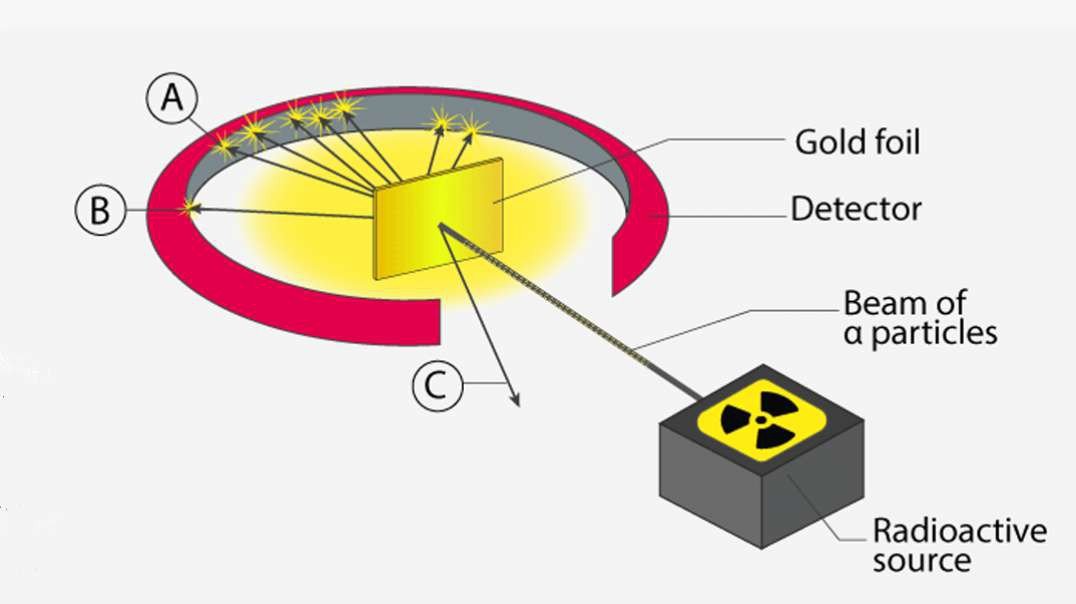
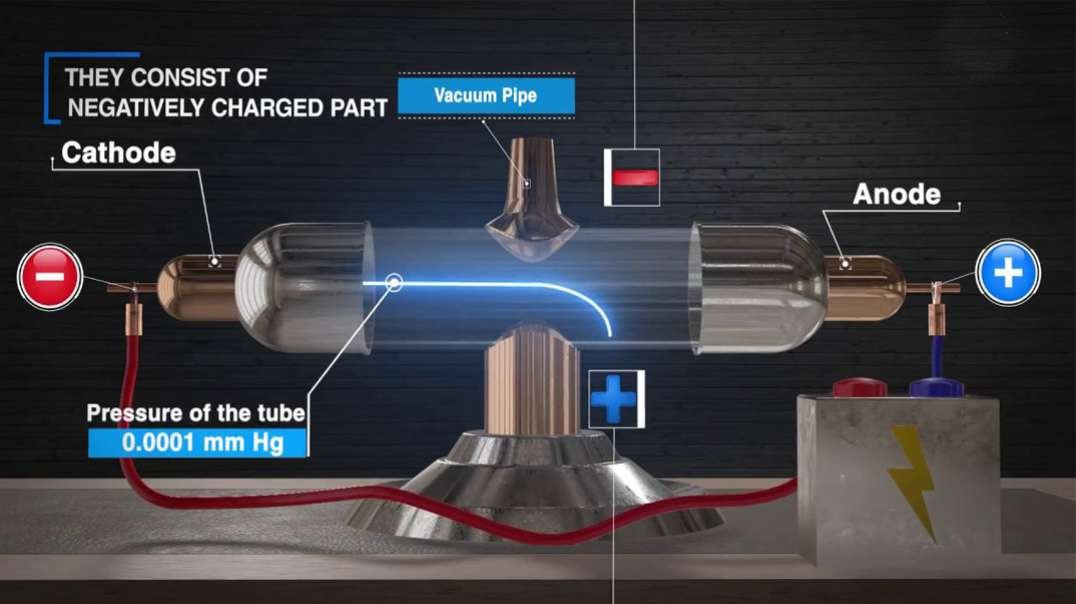
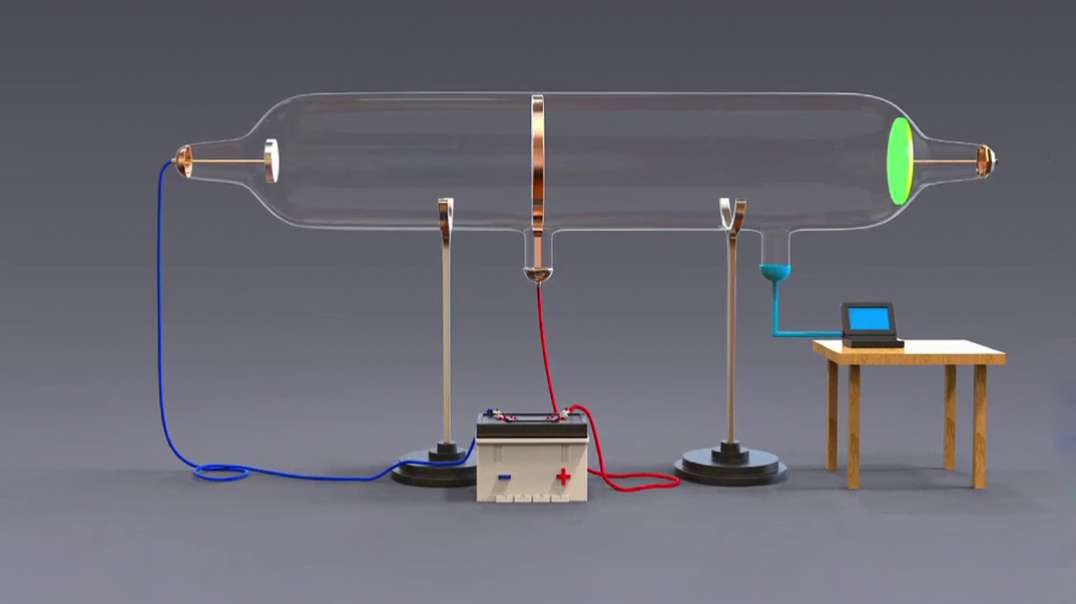
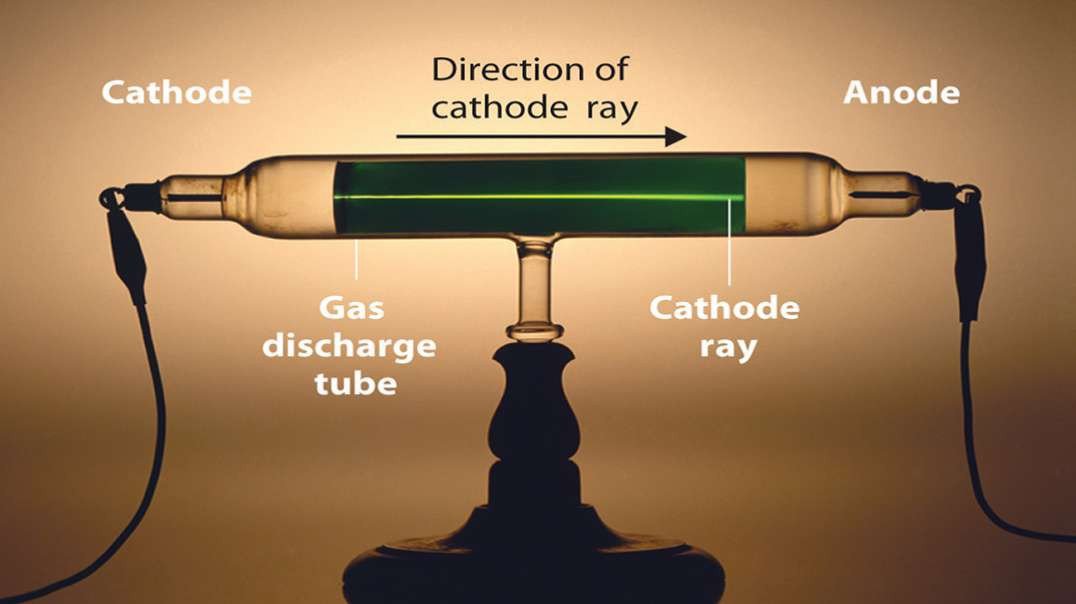
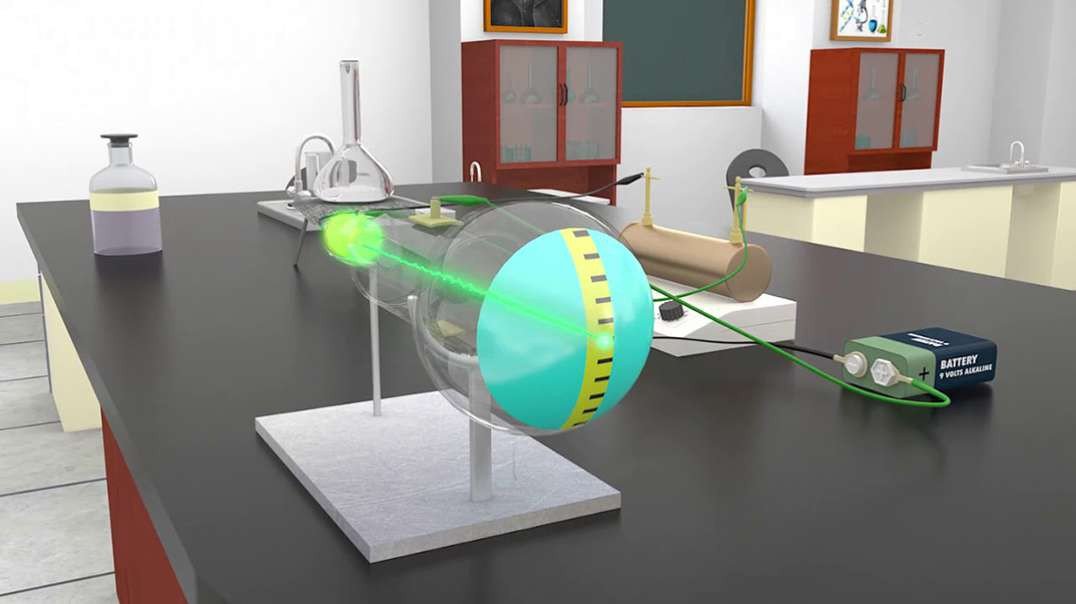
This 3D animation explains the electrolysis of pure alumina that is carried out in a steel tank lined with graphite which acts as the cathode and the hanging carbon electrode act as the anode.
A mixture of Fluorspar and Cryolite was added to Alumina for a better electrolysis process.
The Hall–Heroult process is used at an industrial scale to produce 99.5–99.8% pure aluminum. Aluminum prices lowered because it was hard to extract Aluminium from Alumina before the discovery of this method.
Show more
0 Comments
sort Sort By